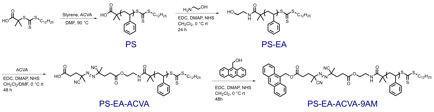Reports: ND753225-ND7: Accelerated Preparation of Polymer-Stabilized Catalytic Nanoparticles
Brent S. Sumerlin, PhD, University of Florida
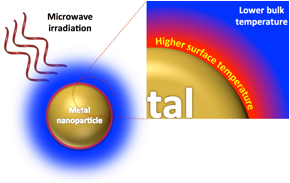
Figure 1. Selective superheating of gold nanoparticles
Polar molecules absorb microwaves and increase bulk temperatures.4 Therefore, it is imperative to choose a non-polar substrate and solvent system to reduce undesired bulk heating events. Polystyrene was prepared via reversible addition-fragmentation chain-transfer polymerization to yield polymers of pre-determined molecular weights and low dispersities (Table 1). Ethanolamine (EA), a thermally labile azo initiator, 4,4′-azobis(4-cyanovaleric acid) (ACVA), and fluorescent reporter molecule, 9-anthracenemethanol (9AM), were conjugated to the macromolecule via sequential EDC coupling reactions (Scheme 1).
Table 1. Polymeric spacers PS 1-4
Entry |
Mn (g mol-1) |
Mw (g mol-1) |
ĐM |
Rg (nm) |
PS 1 |
2 500 |
2 600 |
1.05 |
0.51 |
PS 2 |
6 600 |
6 700 |
1.01 |
0.83 |
PS 3 |
17 100 |
17 800 |
1.04 |
1.34 |
PS 4 |
32 100 |
34 400 |
1.07 |
1.87 |
Scheme 1. Synthesis of functionalized PS
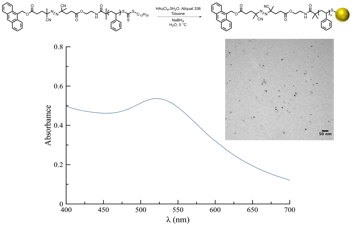
A modified two-phase AuNP synthesis was carried out at 0 °C.5 Utilization of a mild reducing agent prevented ester reduction, while the low reaction temperature protected the sensitive azo linkages. Simultaneous reduction of the gold salt and trithiocarbonate moiety yielded stabilized thiolate-protected AuNPs. A change in color of the organic solution from yellow to deep red indicated formation of AuNPs (D ~ 4-6 nm, Figure 2).
Figure 2. Synthesis, UV-Vis spectrum and Transmission Electron Microscopy micrograph of AuNPs
The fluorescence quenching effect of AuNPs has been extensively studied,6 with significant quenching observed up to 8 nm from the nanoparticle surface.6 Therefore, fluorescent tags attached to the nanoparticle are quenched but will fluoresce upon release into solution. To determine the effect of the AuNPs on unbound 9-anthracenemethanol, a dilute solution of 9-anthracenemethanol was prepared, and the fluorescence was measured in the presence and absence of AuNPs. Due to the markedly low concentration of AuNPs in solution, the probability of interaction with 9-anthracenemethanol is negligible. A quenching effect was not observed, and it was determined that removal of nanoparticles was not necessary prior to taking fluorescence measurements.
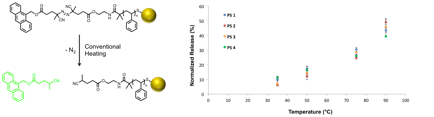
Solutions of decorated gold nanoparticles (0.2 mg/mL) were heated at various temperatures for 1 h, and the increase in fluorescence of each solution was measured immediately thereafter. These measurements were normalized to the complete release of anthracene (90 °C for 18 h) and, the extent of release depends strongly on the oil bath temperature (Figure 4). No significant variation is observed as a function of spacer length, as would be predicted due to the homogeneous heating of the nanoparticle solution under these conditions.
Figure 3. Conventional heating calibration curves for decorated AuNPs
The decorated AuNPs were exposed to microwave irradiation (100 W) for 1 h. The bulk solution temperature (Tbulk) was measured using an external thermocouple, and the vessel was cooled in an ice-water bath. The measured fluorescence value was correlated to the respective calibration curve and the value for the apparent temperature (Tapp) was obtained. Using Equation 1, the increased surface temperature attributed to microwave irradiation of the gold particles (ΔTlocal) was determined.
ΔTlocal = Tapp - Tbulk (1)
Toluene is a theta solvent for polystyrene, and as such, the polymeric ligands should exist in a random coil-like conformation on the surface of the nanoparticles. It can be assumed that the azo moiety will be present at the average radius of gyration (Rg) of the polymer, allowing us to accurately estimate the effective distance between the azo linker and the AuNP surface using Equation 2:
where l![]() is the length of a single styrenic repeat unit (0.252 nm), Mw is the weight average molecular weight of the polymer, and Mo is the repeat unit molecular weight (104.15 g/mol) (Table 1).
is the length of a single styrenic repeat unit (0.252 nm), Mw is the weight average molecular weight of the polymer, and Mo is the repeat unit molecular weight (104.15 g/mol) (Table 1).
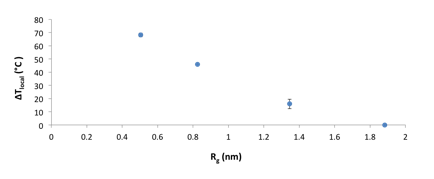
ΔTlocal was plotted as a function of the Rg and the extent of heating shows a strong dependence on the distance between the azo moiety and the nanoparticle surface. In close proximity to the nanoparticle, the temperature increase reaches nearly 70 °C, however, heating effects are not observed 2 nm from the particle surface.
Figure 4. Local temperature as a function of distance
The initial results of this study strongly support the proposed localized superheating of gold nanoparticles under microwave irradiation. Control reactions designed to prove the mechanism of fluorescence release will allow us to confirm these observations were solely the result of localized heating. Further exploration into the effect of microwave power on the extent of heating is necessary and should provide valuable information about this phenomenon. The fundamental information gained in this study will promote increased use of AuNPs combined with microwave irradiation to complete chemical transformations selectively at the nanoparticle surface. With the knowledge gained, it may now be possible to tune our originally proposed system for surface selective polymerization.
1. Keblinski, P.; Cahill, D. G.; Bodapati, A.; Sullivan, C. R.; Taton, T. A. J. Appl. Phys. 2006, 100.
2. Zhang, H.; Xia, H.; Zhao, Y. ACS Macro Lett. 2014, 940-943.
3. Thanaset, T.; Samran, S.; Chanchai, T. Appl. Mech. Mater. 2013, 325-326, 353-358.
4. Brown, S. L.; Rayner, C. M.; Perrier, S. Macromol. Rapid Commun. 2007, 28, 478-483.
5. Siddiqui, M. R. Journal of Chemistry 2012.
6. Schneider, G.; Decher, G.; Nerambourg, N.; Praho, R.; Werts, M. H. V.; Blanchard-Desce, M. Nano Lett. 2006, 6, 530-536.