Reports: DNI751980-DNI7: Hydrolytic Activation of Shape-Memory Elastomers
Christopher Bettinger, PhD, Carnegie Mellon University
Stimuli-responsive polymers play an important role in many biomedical technologies. Light responsive polymers are particularly desirable because the parameters of irradiated light and diverse photoactive chemistries produce a large number of combinations between functional materials and associated stimuli. This project has advanced photoactive chemistries for use in macromolecules for biomedical applications. The project advanced fundamentals of materials selection including choosing photofunctional groups. Synthetic strategies to incorporate these functionalities into polymers and networks with different topologies were also established. Prospective applications of these materials are discussed including programmable matrices for controlled release, dynamic scaffolds for tissue engineering, and functional coatings for medical devices. The project advanced the state of the art in photoresponsive polymers for biomedical applications by addressing many current challenges in this class of materials.
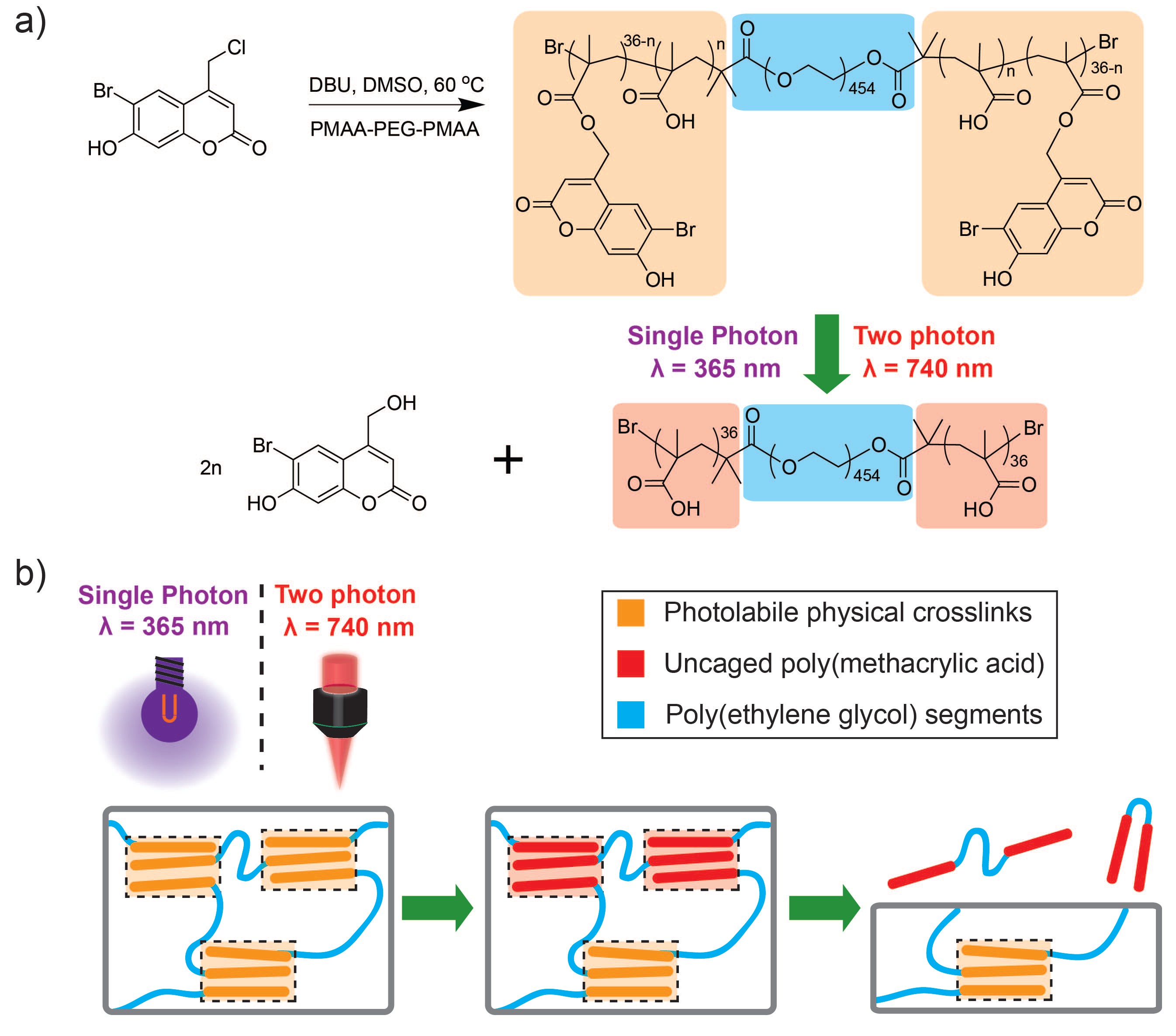
Coumarin-based Photoreconfigurable Networks
Photoreconfigurable hydrogels have been used as light-sensitive biomaterials in biomedical applications including triggerable matrices for controlled drug release,[3] templates for three-dimensional biomolecular patterning[4] and programmable tissue scaffolds.[5] Hydrogels with photocleavable moieties can elucidate the role of physicochemical cues in cell-material responses.[6] Light-induced uncaging is a prevalent strategy to confer photoreconfigurability within crosslinked hydrogels. Next-generation photodegradable biomaterials will benefit from light-sensitive chemistries that can be modulated using benign tissue-transparent wavelengths.[9] Two-photon uncaging of coumarin derivatives using near-IR wavelengths can release bioactive small molecules from both light-sensitive conjugates and micelles.[10] This aspect of the project described the synthesis and characterization of physically crosslinked hydrogel networks composed of triblock copolymers with pendant light-sensitive coumarin moieties, namely, [6-bromo-7-hydroxycoumarin-4-yl]methyl which has been reported with a large two-photon uncaging cross section above 1 GM.[10a]Mechanisms and applications of light-induced network disintegration were also performed.
Briefly, light-sensitive linear amphiphilic ABA triblock copolymers are composed of hydrophilic poly(ethylene glycol) B blocks (PEG, Mw = 20 kDa) and hydrophobic photolabile poly([6-bromo-7-hydroxycoumarin-4-yl]methyl methacrylate) (PBHCMM) A blocks. Poly(methacrylic acid)-PEG-poly(methacrylic acid) (PMAA-PEG-PMAA) triblocks were prepared using atom transfer radical polymerization (ATRP). 6-bromo-4-chloromethyl-7-hydroxycoumarin was conjugated to methacrylic acids via esterification (Figure 1). ATRP synthesis of A blocks using PMAA precursors produces higher molecular weights and smaller PDI compared to coumarin methacrylate. The average degree of polymerization (DOP) of PMAA blocks was 36 for each segment and the degree of esterification by coumarin was 50% as measured by 1H NMR. The esterification efficiency of coumarin on PMAA blocks is lower compared to previous reports that conjugate o-nitrobenzyl halides to PMAA due to increased steric hindrance. Longer reaction times can increase the degree of esterification to >75%. A degree of esterification of 50% was chosen for this work to achieve a balance between robust mechanical properties and accelerated degradation rates of physically crosslinked hydrogels. Minimizing the amount of coumarin can also mitigate the local toxicity risks after uncaging.
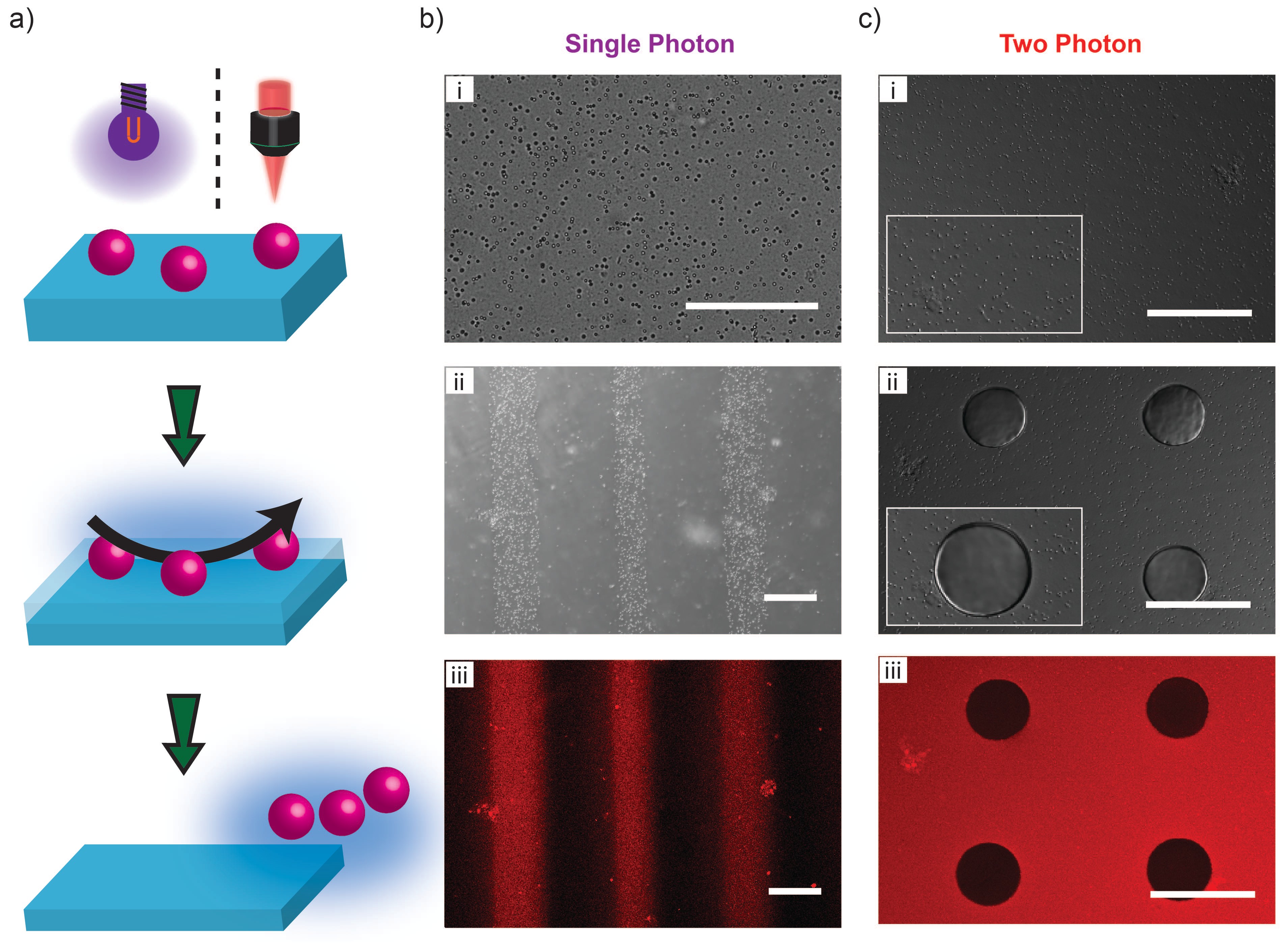
Efficient light-induced remodeling of physically crosslinked hydrogels at tissue transparent wavelengths suggests that implants composed of triblock copolymers can be non-invasively manipulated using light sources from outside the body. Potential applications may include matrices for smart controlled release systems or remotely activated functional surfaces. A potential application as a remotely cleanable surface is examined here using a model material environment. Specifically, photodegradable hydrogel films are prepared into surfaces that can be expunged of adherent microparticles. Fluorescent polystyrene (fPS) microparticles were embedded into the apical region of hydrogels during film preparation. Hydrogels with embedded microparticles were uncaged using single- or two-photon absorption as previously described (Figure 2). Single-photon uncaging was performed with a photomask to demonstrate the selectivity of light-induced surface cleaning. Homogeneously distributed fPS beads were selectively removed in the regions exposed to UV light (Figure 5b). Selectivity in fPS removal using two-photon uncaging was achieved by focusing near-IR laser illumination on the hydrogel surface. Hydrogels with embedded fPS microparticles dedicated for two-photon uncaging were rastered in circular geometries. The disintegrated regions were removed leaving the pristine portion of the gel (Figure 5c). Coumarin-based physically crosslinked hydrogels can potentially serve as medical device coatings that can be cleaned remotely using exogenous light.[15]
Light-induced remodeling of coumarin-based hydrogels offers several notable advantages as a photodegradable polymer network. Physical crosslinks can serve as reservoirs for non-covalent loading of small molecule payloads. Two-photon uncaging permits modulation of network properties at tissue-transparent wavelengths.[16] Finally, PMAA-PEG-PMAA triblock copolymers and coumarin-based by-products of uncaging are potentially less cytotoxic compared to by-products in other photocleavable materials.[17] Poly(MAA-co-BHCMM)-PEG-poly(MAA-co-BHCMM) hydrogels could be processed into functional coatings for medical implants or dynamic material microenvironments to elucidate cell-cell and cell-material interactions.
Structure-Property-Processing Relationships in Photoreconfigurable Networks
Photoreconfigurable physically crosslinked hydrogel networks can be prepared by self-assembly from amphiphilic ABA triblock polymers with photolabile poly(o-nitrobenzyl methacrylate) (PNBMA) A blocks and poly(ethylene glycol) (PEG) B blocks. A detailed structure-property relationship of this class of compounds has yet to be reported. Here, a library of amphiphilic linear PNBMA-b-PEG-b-PNBMA triblock copolymers is synthesized and the physical properties of subsequent hydrogel networks are characterized across two parameters: the degree of polymerization of PNBMA segments and the concentration of PNBMA-b-PEG-b-PNBMA in hydrogel precursor solutions. The storage modulus, photodisintegration kinetics, and swelling ratio are reported. A quantitative model to relate molecular scale uncaging rate of NBMA groups with macroscopic mechanical properties is presented. Hydrogel network parameters including crosslink density and mesh size are also included and compared to covalently crosslinked PEG-diacrylate gels. The concept of reduced swelling ratio is introduced to translate network properties of physically crosslinked photolabile networks formed by self-assembly to covalently crosslinked hydrogels that can be cleaved by uncaging reactions. This revised parameter permits a direct comparison between physically crosslinked networks with covalently crosslinked networks.
Figure: 1
Figure: 2