Reports: ND153385-ND1: Borenium Catalysts for Metal-Free Enantioselective Reductions and Petasis Reactions
Cathleen M. Crudden, Queen's University
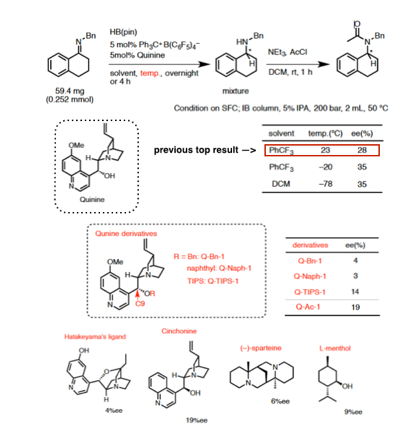
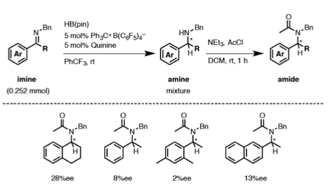
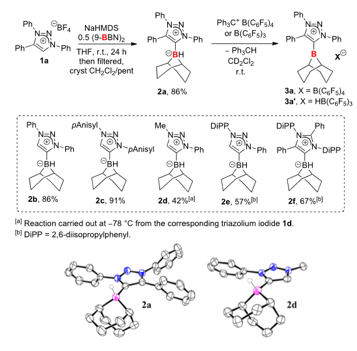
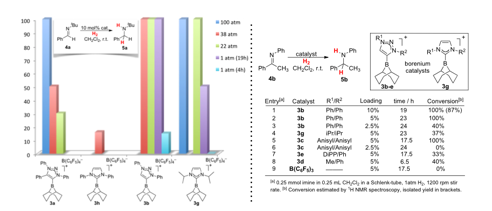
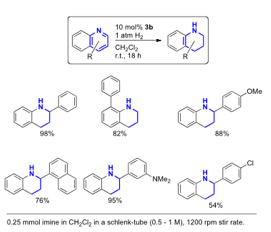
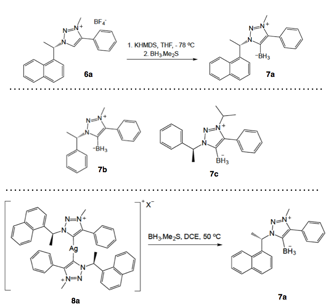
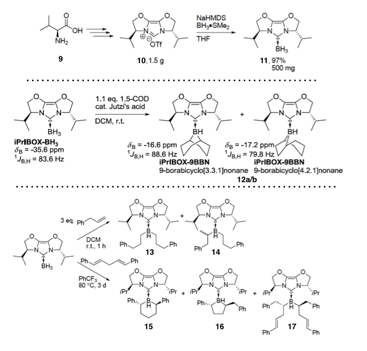
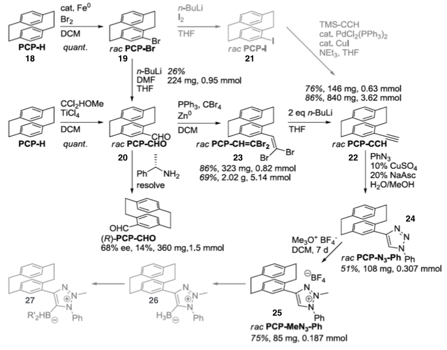
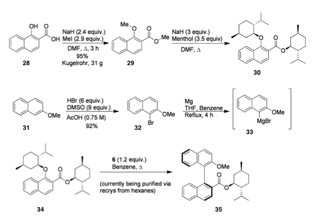
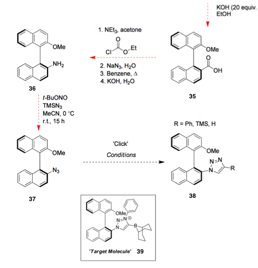
Cathleen M. Crudden, Queen's University










Copyright © American Chemical Society