Reports: UR450664-UR4: Synthesis of Diarylnorbornadiene Derivatives and their Potential Use in Solar to Thermal Energy Conversion
Felix E. Goodson, PhD, West Chester University of Pennsylvania
Karyn Usher, PhD, Metropolitan State University
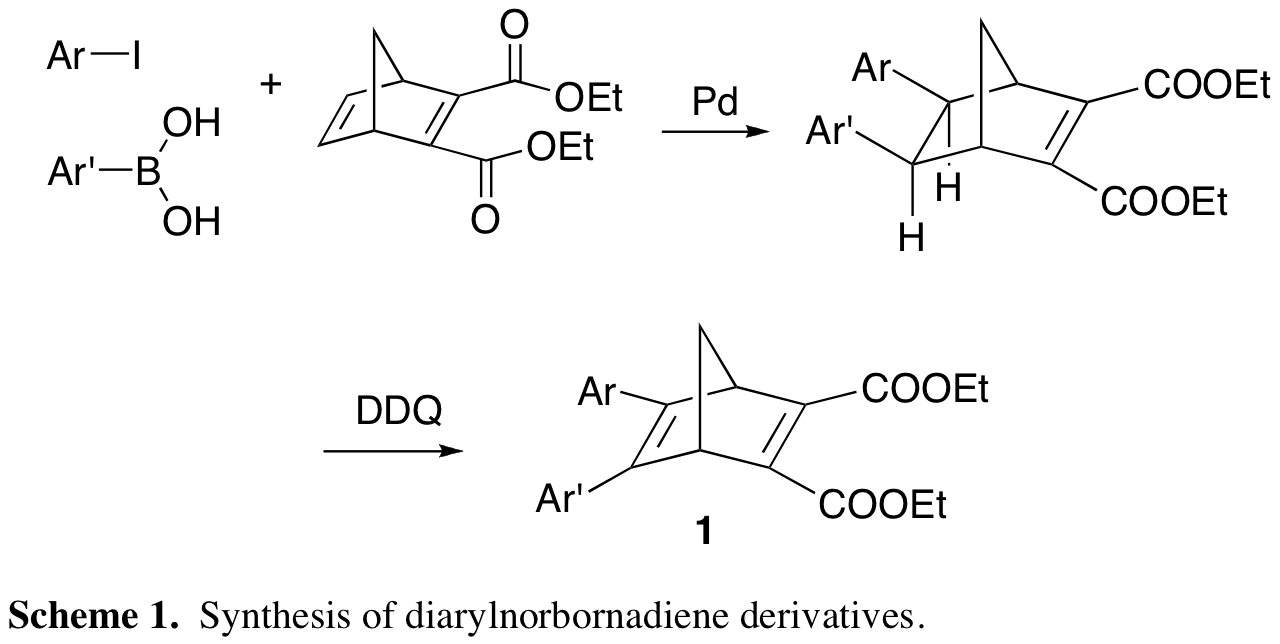
The traditional means of synthesizing these compounds (developed by Hirao in 1984) relied on a multistep route.2 However, we have developed a two-step procedure from readily available starting materials (Scheme 1). In this manner we were able to synthesize and characterize a library of sixteen diarylnorbornadiene derivatives in which groups on one of the aromatic rings have been independently varied.
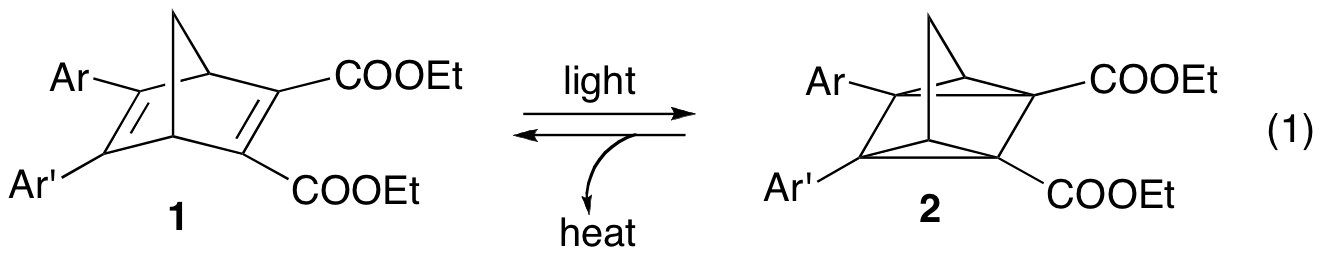
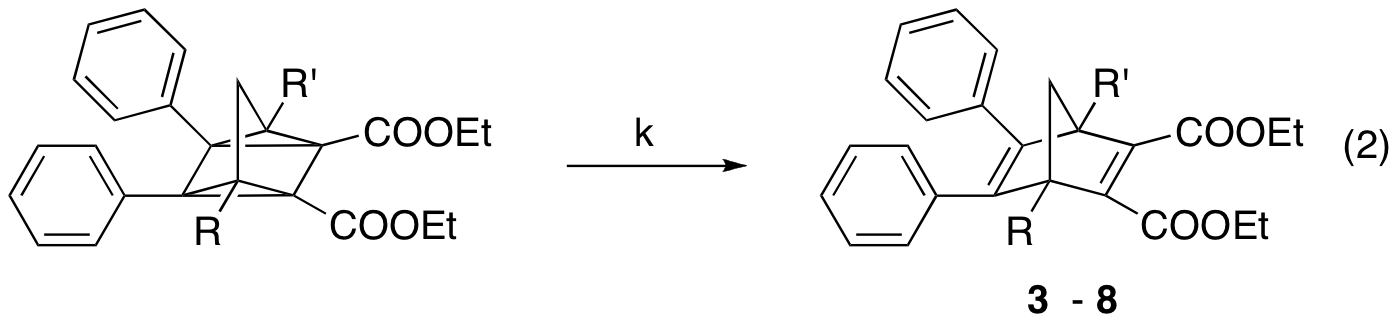
Unfortunately, none of the derivatives in our first library formed a stable quadricyclane, unlike some of the compounds originally presented by Hirao.2 At this point, it became apparent that substitution at other parts of the norbornadiene skeleton would be more likely to generate stable quadricyclanes, and thereby viable candidates for solar energy storage applications. Towards this end, our more recent efforts have resulted in the synthesis of derivatives 3-8, in which we varied the steric hindrance at the bridgehead positions.
We then studied the thermal reversion of the quadricyclane (eq. 2) via differential scanning calorimetry (for onset temperature and heat release of the thermal reversion) and kinetics experiments (for first order rate constants of the thermal reversion). Interestingly, while substituting at the bridgehead position with methyl and ethyl groups provided a significant stabilization effect of the quadricyclanes (ethyl more than methyl), increasing the size of this group further to propyl and butyl had little effect on quadricyclane stability. We were able to increase the stability marginally by incorporating isobutyl groups, suggesting that branching in these positions may have a beneficial effect. All of these derivatives had stored heat contents close to those of examples in the literature.4 It is also worth pointing out that norbornadienes 5 - 8 form quadricyclanes that are stable indefinitely at room temperature.
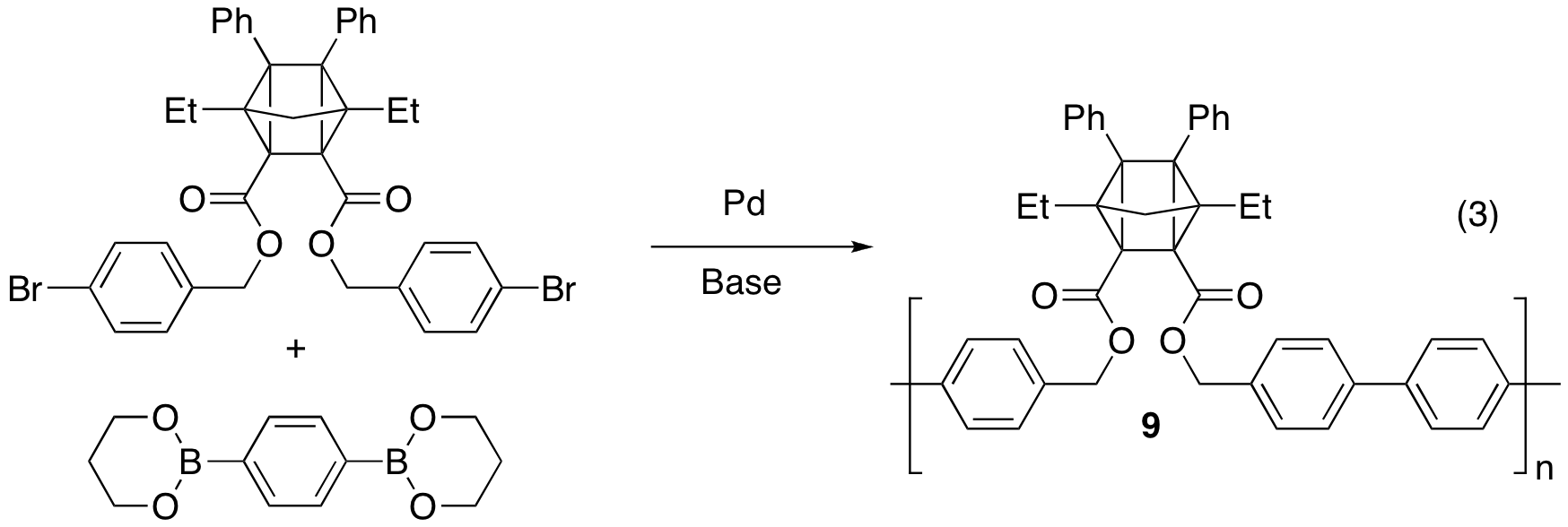
With this success in hand, we next incorporated a stable quadricyclane based on 5 into a polymer (eq. 3). Utilizing optimized Suzuki Polycondensation conditions developed in our laboratory,5 we were able to synthesize polymer 9 with a molecular weight (Mn) close to 50,000 g/mol. Further studies of the thermal properties of this material are currently ongoing.
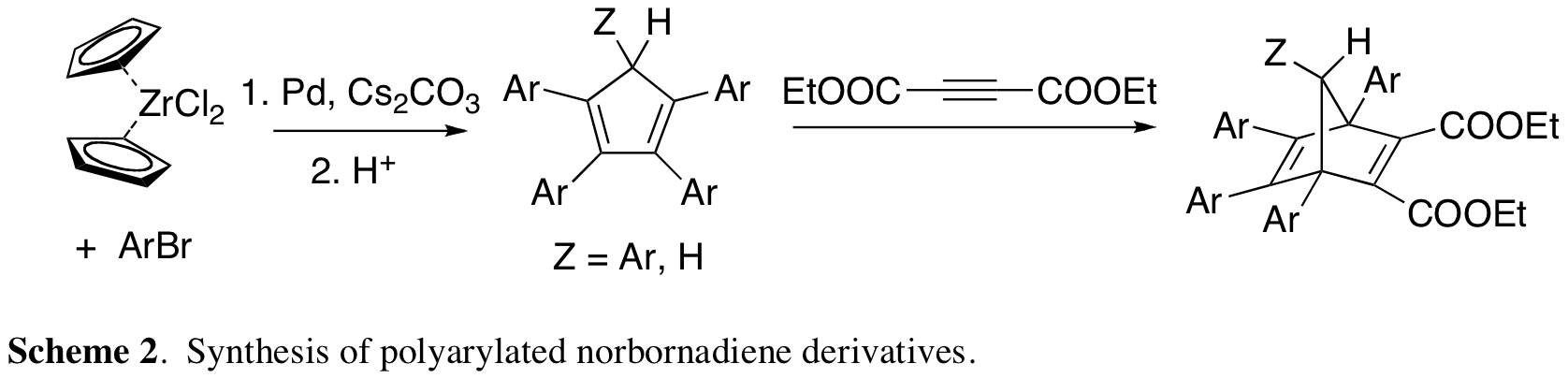
A problem with the compounds mentioned above is that their synthesis was nontrivial. A second project utilized Miura's metallocene arylation reaction6 to synthesize a variety of tetra- and pentaarylated norbornadiene derivatives (Scheme 2). Upon converting these to the quadricyclanes and studying the kinetics of their reversion, we found that the fifth aromatic ring, which substituted at one of the CH2 bridge positions, was vital not only for increasing quadricyclane stability, but also for improving the recovery of norbornadiene at the end of the thermal reversion.
To date, summer research opportunities for eleven undergraduate students have been fully or partially supported by this grant. Of the eight that have already graduated, three are currently pursuing Ph.D. degrees in chemistry and two others are enrolled in graduate programs in other disciplines. Of the three remaining students, two hope to obtain a graduate degree in chemistry, while the third is planning on applying to medical school. Eight of the students have had the opportunity to present their work on this project at recent American Chemical Society national meetings. All eleven of these students have learned skills in organic synthesis, liquid chromatography, and the analysis of kinetics data.
In terms of the impact of this research on the careers of the principal investigators, the technology of chromatography has come a long way since the days of drip columns, which were prevalent in the era when one of us (Dr. Goodson) was in graduate school. The work in this project would not have been possible without the collaboration with Dr. Usher, whose expertise has enabled all of us involved to learn about the advances in liquid chromatography, as well as the skills and instrumentation required to perform the challenging separations. Involvement in this research has had an impact on Dr. Usher's career by introducing her to organic techniques and methodologies that she had not previously experienced. The interaction and collaboration with Dr. Goodson and the undergraduate researchers has been a very positive experience.
References.
1. Dubonosov, A. D.; Bren, V. A.; Chernoivanov, V. A., Russian Chem. Rev. 2002, 71, 917-927, and references cited therin.
2. Hirao, K.; Ando, A.; Hamada, T.; Yonemitsu, O., J. Chem. Soc., Chem. Commun. 1984, 300-302.
3. Shaulis, K. M.; Hoskin, B. L.; Townsend, J. R.; Goodson, F. E.; Incarvito, C. D.; Rheingold, A. L., J. Org. Chem. 2002, 67, 5860-5863.
4. Kabakoff, D. S.; BŸnzli, J.-C. G.; Oth, J. F. M.; Hammond, W. B.; Berson, J. A., J. Am. Chem. Soc. 1975, 97, 1510-1512.
5. Murage, J.; Eddy, J. W.; Zimbalist, J. R.; McIntyre, T. B.; Wagner, Z. R.; Goodson, F. E., Macromolecules, 2008, 41, 7330-7338.
6. Miura, M.; Pivsa-Art, S.; Dyker, G.; Heiermann, J.; Satoh, T.; Nomura, M., Chem. Commun. 1998, 1889-1890.