Reports: UR151819-UR1: Phosphorus-Hydrogen Activation Using Alkynylmetal Complexes: New Methodology for the Preparation of Metallopolymers
Robert A. Stockland, Bucknell University
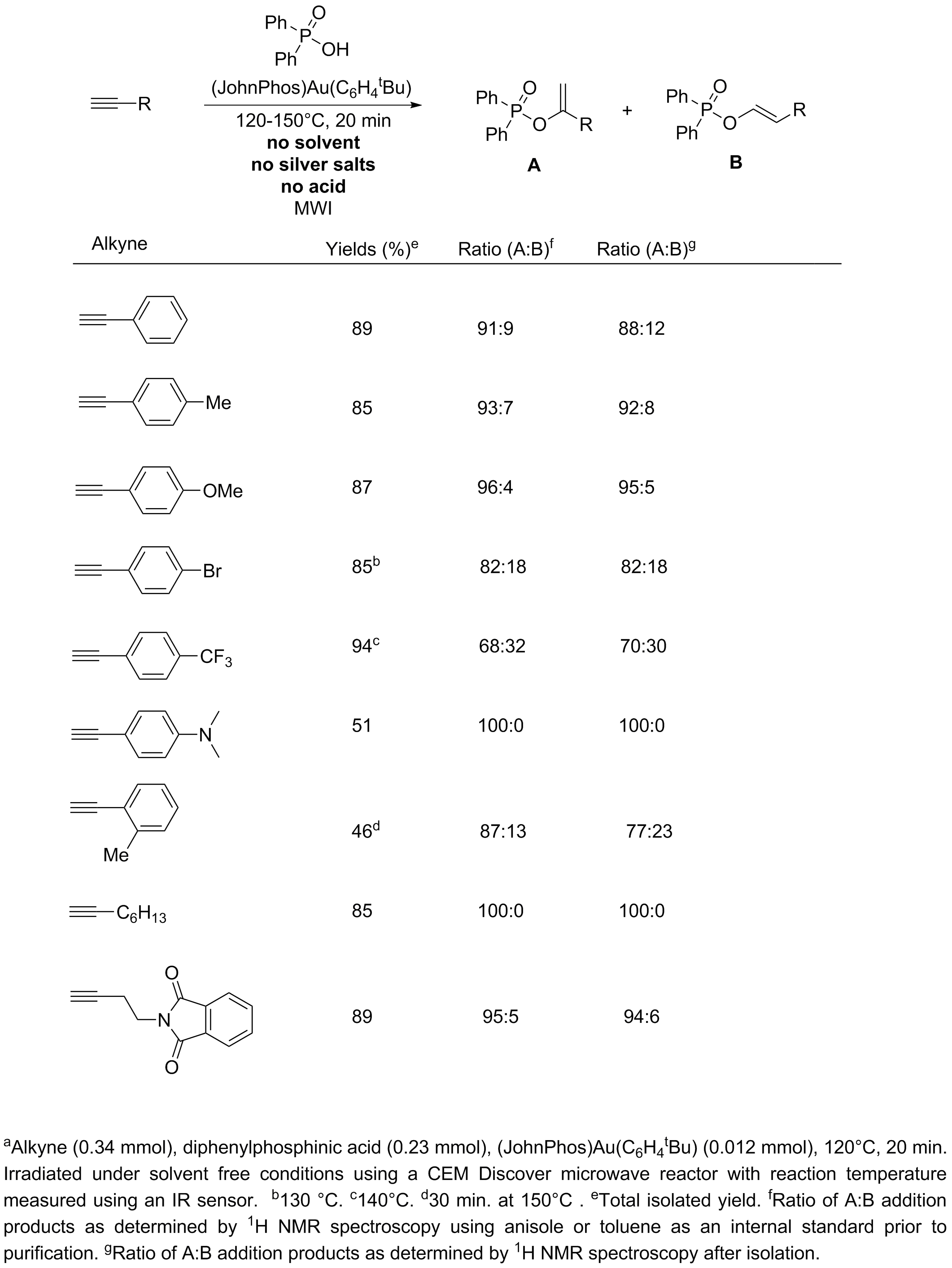
Table 1. Gold catalyzed addition of diphenylphosphinic acid to alkynes.a
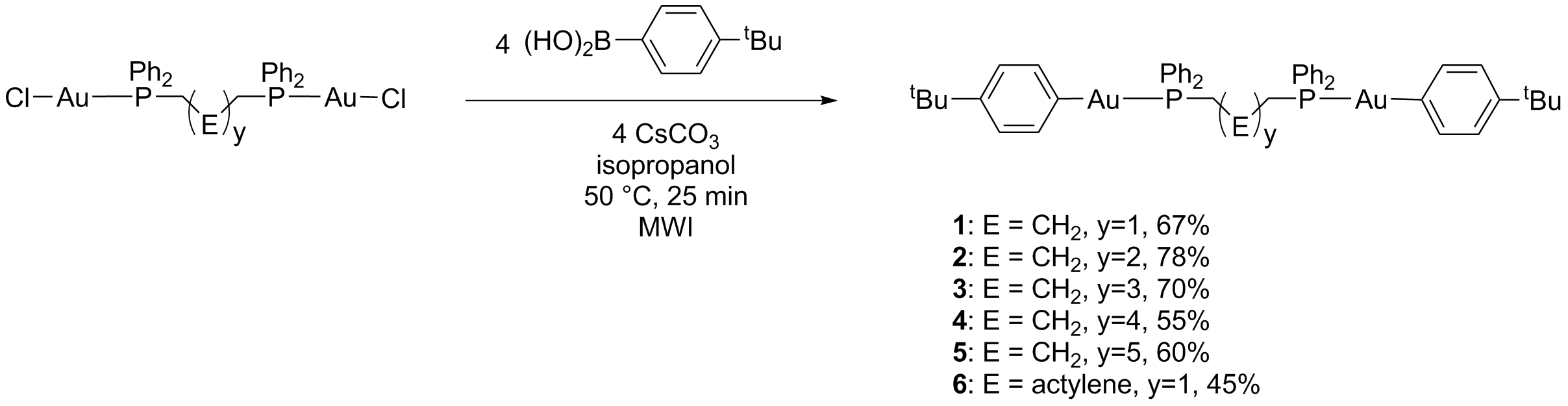
Scheme 1: Preparation of diphosphine ligated diaryldigold building blocks.
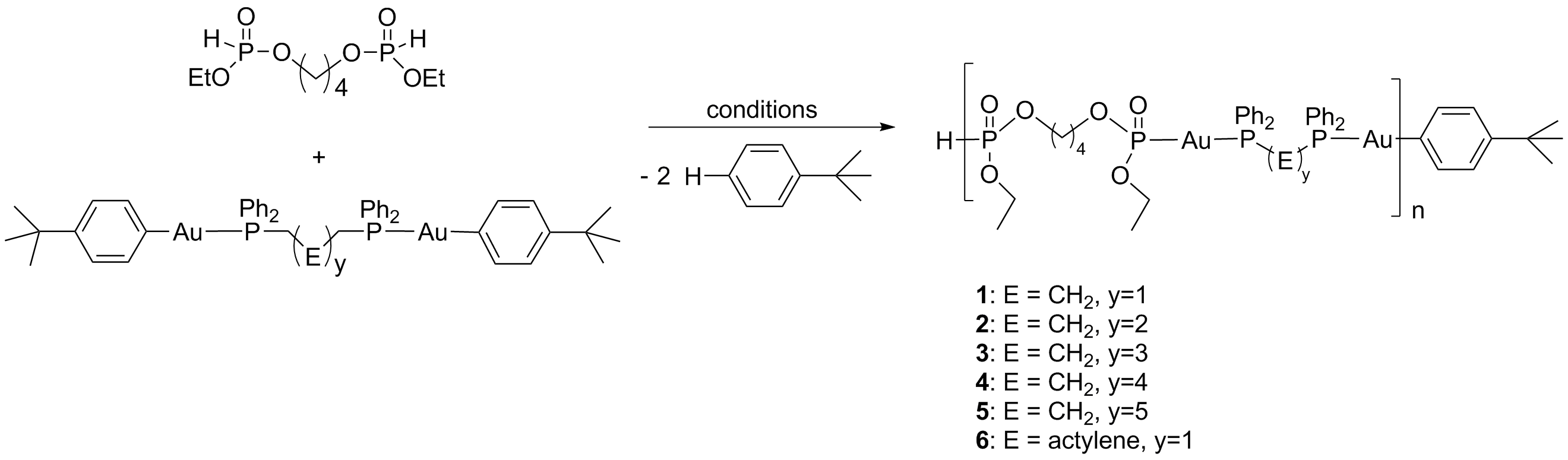
Scheme 2: Synthesis of
metallopolymers through P-H activation reactions.
1) 2) 3)