Reports: DNI151707-DNI1: Novel Catalytic Enantioselective [3,3]-Sigmatropic Rearrangements for the Preparation of Highly Substituted Fused Heterocycles and Biaryls
Laszlo Kurti, PhD, University of Texas Southwestern Medical Center at Dallas
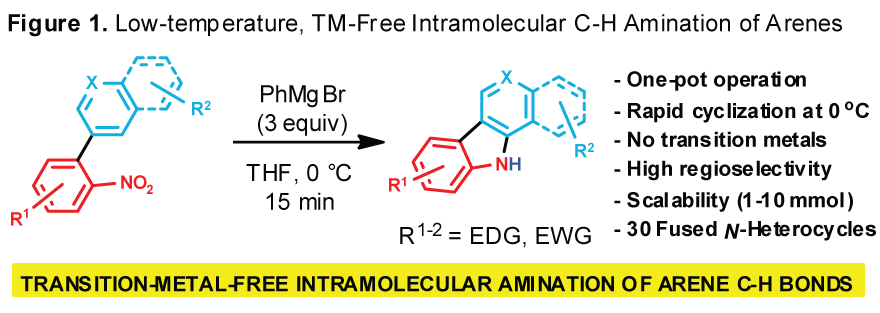
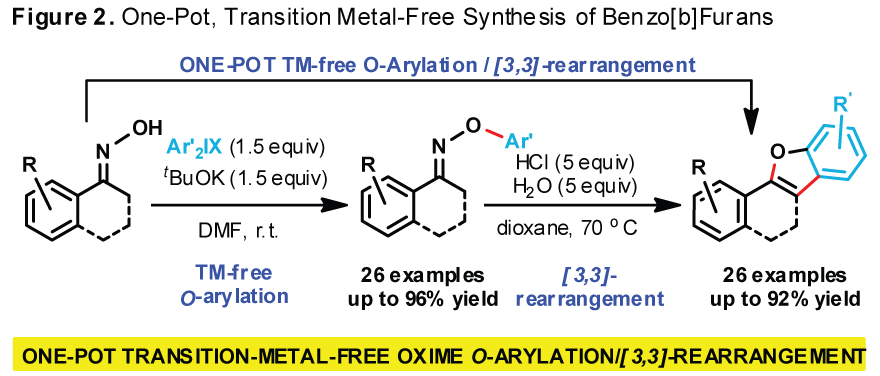
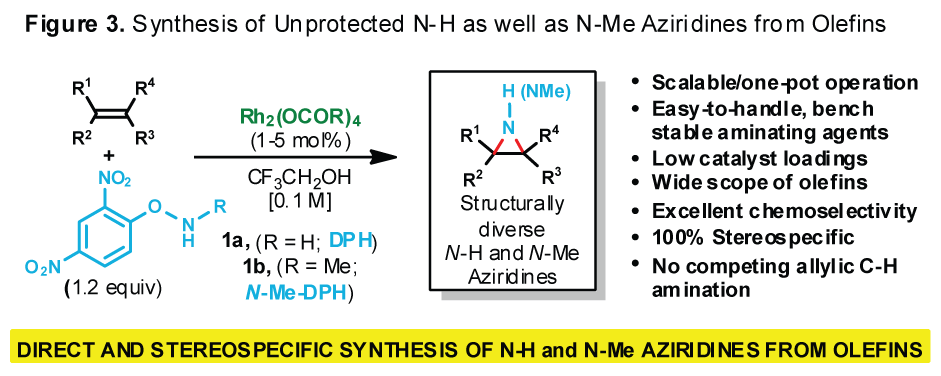
Laszlo Kurti, PhD, University of Texas Southwestern Medical Center at Dallas



Copyright © American Chemical Society