Reports: UR1053498-UR10: Bottom Up Development of a Gas Hydrate Inhibitor Coating
Paul W. Baures, PhD, Keene State College
This project is based on the hypothesis that an inexpensive, durable, and non-toxic polyol-based coating could be developed to inhibit gas hydrate formation in pipelines, building from the knowledge that polyvinyl alcohol and natural antifreeze proteins have both been reported to inhibit hydrate formation. As an initial step towards this end goal, we set out in this first year to synthesize and characterize polyol coatings covalently attached to glass microscope slides, and then determine if these slide surfaces demonstrated antifreeze characteristics. The basis for this approach was that an antifreeze protein attached to a glass microscope slide prevents icing as reported in the literature, making the modified glass surface a useful surrogate for exploring variables such as polyol connectivity, linker chemistry, and functional group density.
Current Results
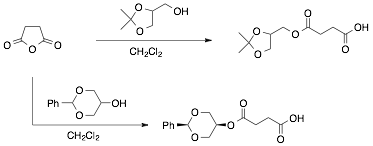
Three undergraduate researchers all majoring in chemistry worked as a team on the project. One student was tasked with synthesizing the designed polyol intermediates as shown in Scheme 1 using methods modified from the literature. The polyols were fully characterized and tested in trial reactions with the conditions used for slide derivatization and polyol deprotection. In this way we were able to optimize in solution the chemistry used for the reactions done on the glass microscope slides.
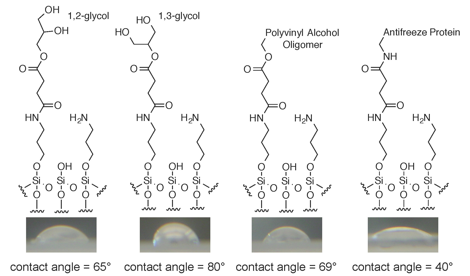
In addition, this student cleaned and subsequently derivatized glass microscope slides with two different aminosilanes following a literature protocol, thereby covalently attaching the polyol intermediates and control compounds to the slides (Figure 1).
This student found that neither FT-IR nor Raman spectroscopy was sensitive enough to provide definitive evidence of the surface modification. To provide such evidence, a second student used a focusing lens and digital camera to take pictures of droplets on the slide surfaces, and then used ImageJ software to determine the contact angles of water droplets (Figure 1). This provided clear evidence of surface modification for most slide surfaces.
Scheme 1
Figure 1. Derivatized slides showing the modifications with 1,2-glycol and 1,3-glycol end groups, a commercial polyvinyl alcohol oligomer, and a semi-pure solution of antifreeze protein isolated from Tenebrio molitor. The completeness of the surface coverage is unknown, so the different stages of the surface modification are shown for each slide. The comparative contact angles for these slides were taken as evidence of successful surface modification.
This second summer undergraduate researcher additionally measured the freezing points and melting points of aqueous solutions containing the synthesized polyols, the polyvinyl alcohol oligomer, and a semi-purified preparation of a mealworm (Tenebrio molitor) antifreeze protein solution. As expected, only the crude protein preparation depressed the freezing point of water in solution consistent with the determined protein concentration and the reported literature freezing point depression value, while not altering the melting point of the frozen solution (a thermal hysteresis known for this antifreeze protein).
The third undergraduate researcher worked on a chamber design (Figure 2) to control temperature and humidity in the range used for icing observations as reported in the literature. This student is continuing to record the condensation and icing behavior of the modified slides and controls in this chamber during the second year of this research. The modified slides were prepared in triplicate and are placed in different locations within the chamber in order to test location as a potential variable. The extent of frosting or ice formation versus simple condensation is determined over the course of 4-6 hours by observation and running a finger gently over the slide at the end of the experiment to determine the extent that droplets are frozen. These experiments are ongoing, with the goal of obtaining enough observations to make a statistically valid conclusion.
Figure 2. View of the temperature and humidity chamber while slides are being cooled and demonstrating observed differences between significant frosting (slide 9; polyvinyl alcohol coating), light frosting (slide 10, glycol-1,3 coating), and predominantly condensate only (slide 1, antifreeze protein coating).
Lastly, we also began forming tetrahydrofuran hydrate (THF hydrate) crystals (Figure 3). These hydrates form just above 0 °C in a proper mix of THF and water (17:1 v/v), using a super-cooled fine copper wire threaded through a glass pipet as a nucleation site. We have grown large crystals of the hydrate by this method, and are preparing to observe modifications to both the growth and stability of these hydrate crystals using the polyols, the polyvinyl alcohol oligomer, and the antifreeze protein solution as a positive control.
Figure 3. Tetrahydrofuran hydrate crystal formed on the tip of a super-cooled copper wire.
In addition to completing the work left from this summer and investigating the THF hydrate formation in year two, we are also preparing to cast films containing polyols that would result in thicker coatings that are easier to characterize and that could be further modified.
Impact
The support from this grant has provided real-world research experience for three undergraduates at different stages of their careers. The project has also been of significant benefit to the PI by providing the financial resources to hire undergraduate researchers during the summer months and pursue this objective. Although no peer-reviewed publications are the product of this work to date, each of the three students presented the results of their summer research at the NH-INBRE conference. The students are planning to take their posters to the American Chemical Society National Meeting in Denver, CO, in March of 2015.