Reports: DNI751850-DNI7: N-Alkyl Urea Peptoid Oligomers as a New Type of Self-Assembling, Highly Versatile Soft Materials for Applications Involving Organogelators
Neil Ayres, PhD, University of Cincinnati
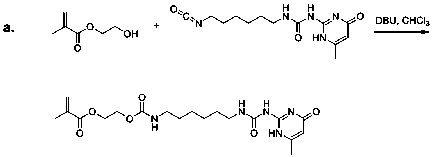
A UPy containing monomer (UPyMA) was previous synthesized by Long's group via the reaction of 2-amino-4-hydroxy-6-methylpyrimidine and 2-isocyanatoethyl methacrylate. In the previous year of our grant support, we found UPy moiety possessed poor solubility. In order to increase the solubility of our UPy monomer, we designed an alternative synthesis to Long's method. Our UPy containing methacrylate monomer (UPyEMA) was synthesized via reaction of a 2-hydroxyl ethyl methacrylate and a UPy-functionalized isocyanate in 70% yield (Scheme 1a). Comparing our monomer with that of Long's, the UPyEMA synthesized by our method contains an extra hexyl carbon chain and a carbamate functional group to increase solubility.
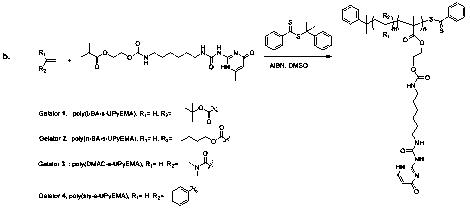
Scheme 1. (a). Synthesis of UPyEMA. (b). Synthesis of gelators 1-4.
We used UPyEMA as a co-monomer for the synthesis of four copolymers using four other monomers. The four monomers chosen were n-butyl acrylate (n-BA), t-butyl acrylate (t-BA), N,N-dimethyl acrylamide (DMAc), and styrene (sty). Tg values of polymers from these monomers are -54 oC, 45-100 oC, 79 oC, and 110 oC respectively. Inverted vials showing gels formed from choloroform solutions of gelators 1-4 (from left to right) at their critical gelation concentration.
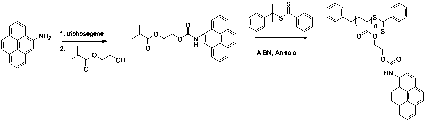
A pyrene containing monomer was prepared so the polymeric organogelators can be used in potential energy transfer and up-conversion applications. The molecule 1-amino pyrene was treated with triphosgene followed by addition of HEMA to produce the monomer PyEMA in 50 % yield (Scheme 2).
Scheme 2. Synthesis of the pyrene based monomer PyEMA and its subsequent polymerization with CDB.
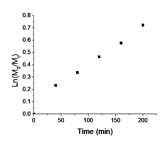
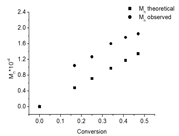
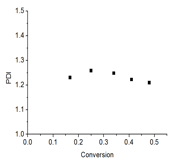
The kinetics of PyEMA polymerization was studied to show the monomer is compatible with controlled/living polymerization. CDB was chosen as the chain transfer agent as it has demonstrated control over methacrylate polymerizations. The kinetics of polymerization were linear over the time-length of the reaction and a conversion of approximately 50%. Figure 2 also shows plots of Mn and polymer dispersity index (PDI) of the polymers vs. monomer conversion. The Mn increased linearly with monomer conversion and the PDIs were narrow (Mw/Mn < 1.2) during the polymerization. These results indicate the RAFT polymerization of PyEMA is controlled. The observed Mn is slightly higher than theoretical probably due to consumption of the RAFT agent in early termination.
a.
b.
c.
d.
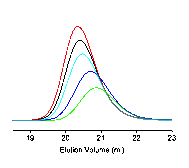
Figure 2. (a). kinetics plot of RAFT polymerization of PyEMA with AIBN as initiator and CDB as RAFT agent at 80 °C in anisole; (b) Mn vs. conversion plot; (c) the PDI vs. conversion plot; (d) GPC traces of polyPyEMA during kinetic studies; From right to left: 40, 80, 120, 160, 200 mins.
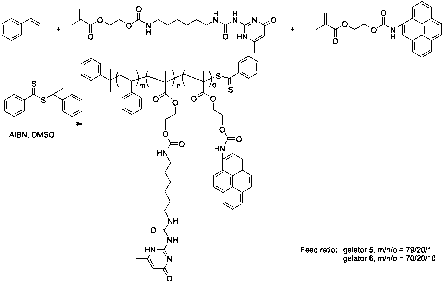
We prepared two statistical copolymer organogelators containing PyEMA. The PyEMA feed ratio was either 1% (gelator 5) or 10% (gelator 6) (Scheme 3). We chose polystyrene as the comonomer. The polymerizations were carried out in DMSO at 100 °C for 32 h. The polymers were precipitated into methanol, as both unreacted UPyEMA and PyEMA are soluble in methanol. GPC analysis showed gelator 5 and 6 possessed Mn values of 17 900 g/mol and 19 600 g/mol respectively, with PDIs around 1.5.
Scheme 3. Synthesis of pyrene containing gelator 5 and 6.
The molecular formula of gelator 5 was calculated as poly(Sty100-co-UPyEMA17-co-PyEMA2) using NMR spectroscopy. The same method was applied to determine the formula of gelator 6 as poly(Sty87-co-UPyEMA13-co-PyEMA13). Statistical copolymerization of PyEMA, UPyEMA, and styrene using RAFT yielded stable transparent gels in chloroform. The critical gelation concentration (CGC) of gelator 5 and 6 in chloroform was determined as 6.3 % weight percent and 9.2 % weight percent. Dilute solutions of 5 and 6 were probed with UV-Vis spectroscopy to reveal the UV absorption and fluorescence emission of the materials. We measured solution of 6 at 1/10 fold of solution of 5 since the incorporation of pyrene moieties of 6 was much higher than 5. Both 5 and 6 showed characteristic pyrene absorption around 350 nm and fluorescence emission between 380-450 nm.
In summary our accomplishments this year have been to make a new set of polymeric organogelators, building off of our findings from the previous year with small molecule organogelators. This has lead to the synthesis of a novel gelled system containing anthracene that possesses potential light upconversion properties. We are currently investigating these properties with our collaborators.