Reports: ND353315-ND3: Rational Synthesis of Iridium(III)-Alkane Complexes
Thomas Gerald Gray, Case Western Reserve University
Energy resources of the future will emit lesser quantities of CO2into the atmosphere than those used in former years. Alkanes have the lowest carbon footprint per mole of hydrocarbons, and methane, the lowest of all. Experimentation is needed for metal complexes to realize their potential in the production and refining of petroleum and natural gas. Alkane complexes durable enough for solution-phase characterization are rare. Those encountered to date have attracted attention.
Thermally stable alkane σ-complexes are sought for iridium(III). Octahedral iridium(III) is low-spin d6, and hence substitutionally inert. Rates of aquo ligand exchange on [Ir(H2O)6]3+ show it to be the most inert metal ion, with a residence time for bound water of ca. 300 years.[1] The high positive charge of this ion reinforces its inertness. Charged iridium(III) complexes are being investigated as precursors to metastable alkane complexes.
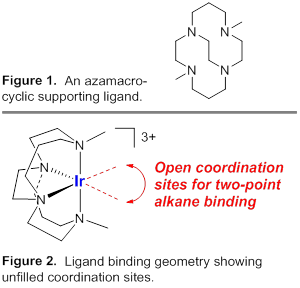
A library of iridium(III) complexes is being synthesized with neutral, tetradentate ligands. Pseudo-octahedral complexes are sought where alkanes bind in a two-point manner. That is, the alkane ligand occupies two sites in an octahedral coordination sphere. One such ligand is the cross-bridged cyclam appearing in Figures 1 and 2. The Gray research group is developing transformations of iridium(III)-halide precursors that afford σ-alkane complexes. Current work emphasizes the synthesis of new halide complexes, their transformations to metal alkane complexes, and characterization of new species.
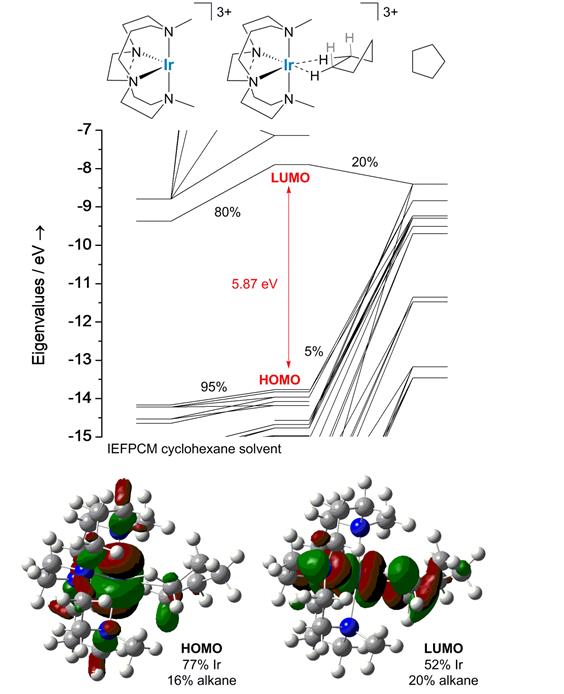
Density-functional theory calculations suggest that binding enthalpies of alkanes to coordinatively unsaturated iridium(III) can exceed 30 kcal/mol. The calculations also suggest that the highest occupied orbitals lie on the alkane fragment, whereas the lowest unoccupied orbital is metal-centered. Figure 3 shows a frontier Kohn-Sham orbital energy level diagram of a representative target. The bound alkane is cyclopentane, which attaches in a pseudo-bidentate structure. That is, the alkane takes up two coordination sites. Binding through two sites, instead of one, impedes alkane loss.
Impact of the Research.This work is enabling the design of molecular alkane σ-complexes that are kinetically stable near room temperature. Although metal-alkane σ-complexes can be synthesized rationally, their lability usually defeats characterization except at low temperatures. This PRF-supported research seeks robust metal-alkane complexes. The choice of iridium(III) is deliberate. Ir(III) is the most substitutionally inert—and hence the "stickiest"—metal ion yet identified. Reliable access to thermally stable alkane σ-complexes will foster the development of more efficient methods to refine crude oil and natural gas.
ACS PRF NDI support has permitted the Gray laboratory to begin a major new project in metal-alkane complexes. Essential preliminary data are being gathered for eventual federal funding. One graduate research assistant has been supported with these funds. This student is being trained ain Schlenk and inert-atmosphere techniques. The student’s training was made possible by PRF support.
Figure 3.Frontier Kohn-Sham orbital energy level of a σ-cyclopentane complex. Depictions of orbitals and compositions in terms of fragment orbital densities appear at right.
[1] Cusanelli, A.; Frey, U.; Richens, D. T.; Merbach, A. E. "The Slowest Water Exchange at a Homoleptic Mononuclear Metal Center: Variable-Temperature and Variable-Pressure 17O NMR Study on [Ir(H2O)6]3+," J. Am. Chem. Soc. 1996, 118, 5265–5271