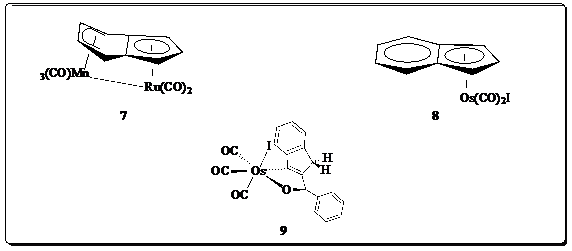Reports: UR351747-UR3: Hydrogen Production via Homogeneous Catalysis: Investigations of the Water-Gas-Shift Reaction
Jason S. D'Acchioli, PhD, University of Wisconsin (Stevens Point)
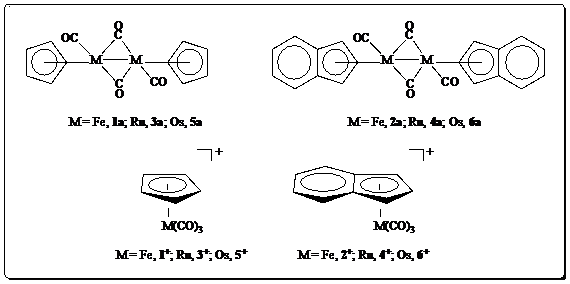
1. Introduction. The synthesis and characterization of a series of Group 8 organometallic complexes (Figure 1) has been undertaken in order to probe their involvement in the so-called water-gas-shift (WGS) reaction (Figure 2). We have shown in prior work that 4+ is active in the WGS reaction.1
Figure 1. Target complexes under investigation.
Figure 2. The water-gas-shift reaction. 2. Progress. Our attention over the past year was
focused on four areas: 1) synthesizing 5+ or an
analogue, 2) attempting to synthesize 6+ or an analogue, 3)
attempting to synthesize the heterobimetallic complex 7 (Figure 3), and
4) further investigating the chemistry of 4a. Each of these is described
in turn.
Complexes 5+ and 6+
have never been synthesized. Complex 8, related to 5+
but with an Os(CO)2I moiety, has been synthesized.2 We
attempted a modified synthesis of 8 (Figure 3) using a specially
designed heavy-walled Schlenk flask. The reaction, run in toluene as an alternative
to benzene, yielded compound 9 (Figure 3) as the major product. We have
verified 9 from single-crystal X-ray diffraction studies, 1H
and 13C NMR spectra, and IR analysis. We are still investigating
ways of synthesizing 8.
Compound 7 is intriguing because it may
provide a way of stemming formation of dimer 4a in the WGS reaction.
Sweigart and coworkers have previously synthesized the iron analogue of
compound 7.3 We have some IR (KBr pellet) evidence of the
formation of 7, but solubility issues have plagued further analysis.
This is an active area of investigation.
Dimer 4a is used as the starting material
in the production of 4+. Once 4+ has been
reduced in the presence of base, though, 4a is formed again. Our prior
thinking was that, if 4+ were the catalyst, then 4a is
the product of the WGS reaction after catalyst “poisoning”. We are currently
investigation whether 4a can be used directly as a WGS catalyst.
Figure 3. Complexes considered
in this report. 3. Impact on PI. The PRF grant has had a significant
impact on the PI's career. First, it has allowed me to attract, and retain,
outstanding undergraduate students. Second, it has allowed me to maintain my
fruitful collaboration with Professor Arnold Rheingold (UCSD), who is
determining structure through single-crystal diffraction. Finally, it has
allowed me to establish collaboration with Professor Eric Watson (Seattle
University), who is supplying one of the starting materials for synthesizing 7.
4. Impact on Undergraduates. The students working on
this project are two female undergraduates newly recruited from courses taught
by the PI in the chemistry department’s general chemistry sequence. Both
students are fast learners and exceptionally industrious, and have quickly
adapted to the challenges of Schlenk-work. Both students are looking to present
their results at the ACS National Meeting in Denver, Colorado, in 2015.
5. References. 1. Badger, R. C.; D’Acchioli, J. S.;
Oudenhoven, T. A.; Walder, B. J. Organometallics. 2010, 29,
1061-1063.
2. Rosenberg, S.; Herlinger, A. W.;
Mahoney, W. S.; Geoffroy, G. L.; Hembre, R. T.; Birdwhistell, K. R.; Norton,
J. Inorg. Synth. 1989, 25, 187-192.
3. Sun, S.; Dullaghan, C. A.; Carpenter,
G. B.; Rieger, A. L.; Rieger, P. H.; Sweigart, D. A. Angew. Chem. Int. Ed. 1995,
34, 2540-2542.