Reports: ND653320-ND6: Photochemistry and Unimolecular Dynamics of Criegee Intermediates
Marsha I. Lester, PhD, University of Pennsylvania
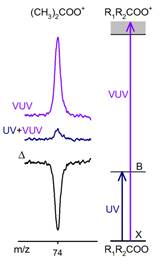
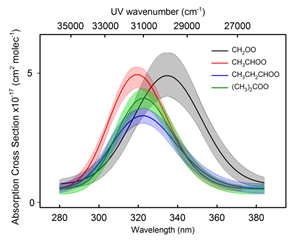
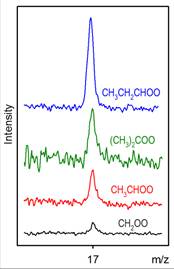

Marsha I. Lester, PhD, University of Pennsylvania




Copyright © American Chemical Society