Reports: ND252142-ND2: A Potential Fungal Contribution to the Selective Preservation of Long-chain Hydrocarbon Functionality in Soils and Sediments
Neal E. Blair, Ph.D., Northwestern University
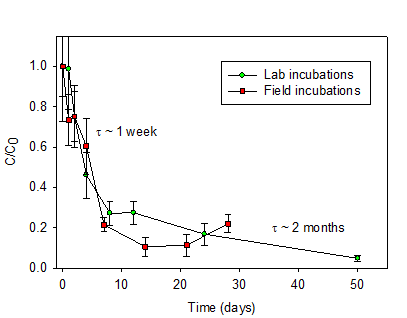
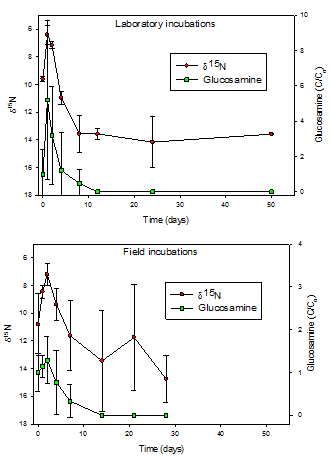
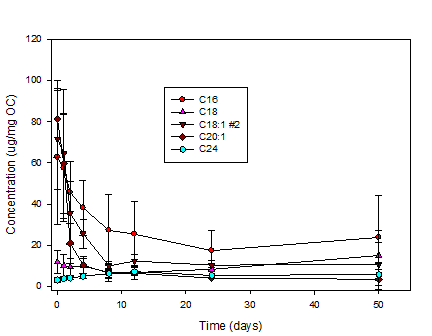
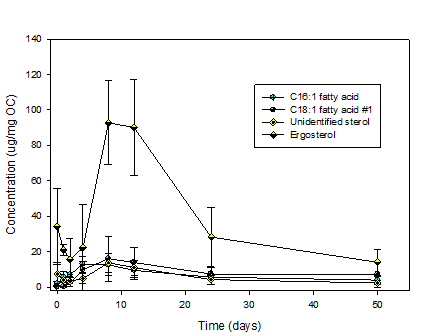
Neal E. Blair, Ph.D., Northwestern University




Copyright © American Chemical Society