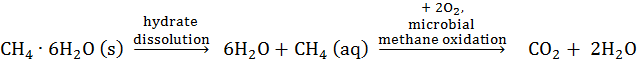Reports: DNI252128-DNI2: Understanding Factors that Control Gas Hydrate Stability: Does Microbial Activity Induce Gas Hydrate Dissolution?
Laura Lapham, University of Maryland Center for Environmental Science
where CH4·6H20(s) is pure methane hydrate, CH4 is dissolved CH4 and CO2 is carbon dioxide formed by aerobic methane oxidation. Methanotrophs oxidize methane, pulling equation 1 further to the right, enhancing hydrate dissolution. The approach was to synthesize methane hydrate in the laboratory and expose it to a pure culture of aerobic methanotrophs, under high pressure and low temperature. This culture had not been grown under these extreme conditions, so our first task was to determine if it would grow under hydrate formation conditions. Growth was monitored via methane concentrations, methane stable isotopes, and cell turbidity (methods described in year 1 report). In year 1, the pure culture of methanotrophs (Methylmicrobium Album) grew under high pressure, 34 atm; but not low temperature. The goal for year 2 was to determine how to grow the culture at low temperatures (results described below). While we obtained promising results, we have not been able to grow the culture at low temperatures. This result has precluded us from carrying out the main hydrate experiment to determine if microbes dissolve hydrate. With the no-cost extension of this grant, we are testing other microbial cultures, obtained from the environment, to determine if they will grow at low temperatures. The primary success of this project will be the graduation of Lauren Gelesh in December 2014; funded on PRF funds. Her thesis will be available to PRF.
Details of experimental results
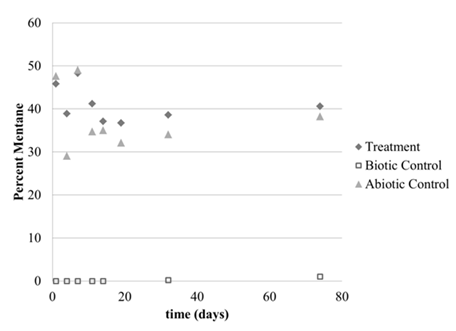
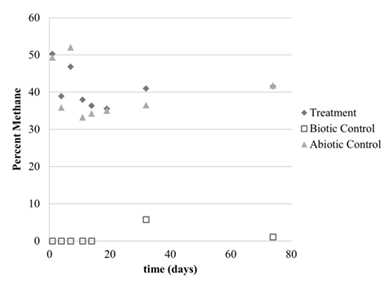
The first 3 months of year 2 were spent working with the culture at low temperatures. The culture was grown at 10°C and 1 atm (Figure 1) and 1°C and 1 atm (Figure 2). There was an initial drop in concentrations during the first 10 days, but the lack of continued methane depletion suggested no growth over the 2 month time period in both experiments. There was also no change in optical density for both experiments (data not shown), so we concluded there was no cell growth at these low temperatures. These experiments were carried out in glass vials.
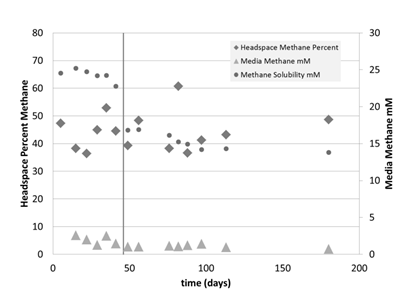
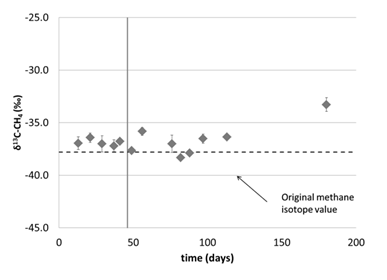
To simulate the growth conditions for the hydrate experiment, the culture was then grown in the stainless steel pressure chamber (Figure 3). The experiment began at 15°C and 34 atm but no growth was seen after 46 days. To see if the culture was still viable, the temperature was increased to 30°C at the same pressure. No change in methane concentrations over time resulted (Figure 3). However, the stable carbon isotopic signature of the methane (δ13C-CH4) did get heavier towards the end of the 180 day time series, indicating methane oxidation (Figure 4).
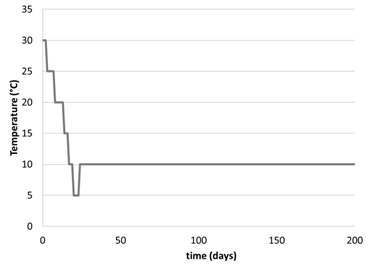
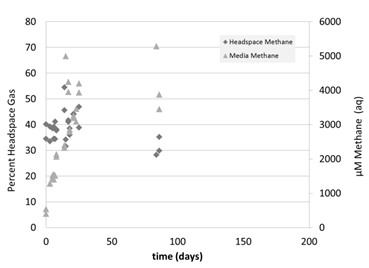
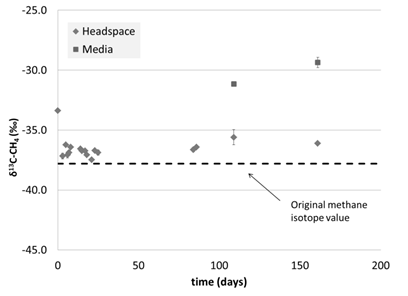
We then tested the idea that the initial shock of going from relatively warm conditions to cold conditions within a few hours was deleterious for the microbes by gradually decreasing temperature over the course of 1 month (Figure 5). Every ~4 days, the temperature was dropped by 5°C, until 10°C (Figure 5). Methane concentration initially increased, due to the increased solubility of methane at colder temperatures (Figure 6). Beyond this, only one concentration measurement was taken due to analytical issues. It was promising that the δ13C-CH4 values increased over time in the media, suggesting that the microbes were oxidizing methane (Figure 7). Future studies will focus on measuring the dissolved phase as the more sensitive measure of growth.
Enrichment experiment
Since the pure culture was reluctant to grow at gas hydrate formation temperatures, bottom water from a Gulf-of-Mexico hydrate seep was enriched for aerobic methane oxidizers. Water was placed into glass serum vials, given a headspace of 50% methane: 50% air, and left at 4°C in the dark for 15 months. Over this time, growth was checked by visual observation of turbidity. There was no change over the time series, suggesting that we did not enrich for methane oxidizing bacteria. However, in light of our year 2 results, δ13C-CH4 will be measured during the no-cost extension.
Synthesizing hydrate
Gas hydrate was successfully synthesized by adding a pure methane headspace to 300mL of microbial media (data not shown) but no experiments were carried out with this hydrate. A second attempt to synthesize hydrate using filtered seawater from a Gulf of Mexico methane seep was carried out. The hydrate formed after several weeks (usually it only takes days). After a month, we attempted to flush the methane headspace away to carry out a dissolution experiment (this requires dropping pressures to atmospheric), and the hydrate immediately dissociated. This hydrate had a very different composition than others grown in the lab (1), and follow-up experiments will need to be conducted.
References
1. Lapham, L. L., et al., 2014. Geochimica et Cosmochimica Acta 125 492-503.
Figure 1: Percent methane headspace concentrations at 10°C and 1 atm in the treatment (50% methane:50% air), biotic control (media inoculated but no methane added to headspace) and abiotic control (media not inoculated but was given 50% methane:50% air headspace).
Figure 2: Percent methane headspace concentrations at 1°C and 1 atm (see figure 1 for legend explanation).
Figure 3: Headspace (%, left) and media (mM, right) methane concentrations containing culture from the pressure chamber at 15°C and 34 atm. After 46 days, the temperature was increased to 30°C, indicated by the vertical line. Methane solubility (mM, right) is also shown (dots).
Figure 4. δ13C-CH4 values in the headspace from the pressure chamber at 15°C and 34 atm. After 46 days, the temperature was increased to 30°C, indicated by the vertical line.
Figure 5: Temperature plot for experiment carried out in pressure chamber where culture was monitored at 34 atm.
Figure 6: Methane concentrations in headspace (%, left) and dissolved in media (µM, right) of the pressure chamber experiment at variable temperatures and 34 atm.
Figure 7: Headspace and media δ13C-CH4 for pressure chamber experiment at variable temperatures and 34 atm. Error bars show standard error on duplicate measurements.