Reports: DNI552089-DNI5: Metal Clusters Sequestered in Micropores of Hierarchical Lamellar Zeolites: Protected Active Sites and Enhanced Mass Transport for Heteroatom-Tolerant Catalysts
Dongxia Liu, University of Maryland
(A) (B) (C)
(D) (E) (F)
Figure 1. SEM images showing morphologies of Mo loaded lamellar MFI catalysts: (A) Mo-MFI-10/0, (B) Mo-MFI-10/2, (C) Mo-MFI-10/5, (D) Mo-MFI-10/8, (E) Mo-MFI-10/12, and (F) Mo-MFI-10/36, respectively.
Table 1. Composition and acidity of the lamellar
MFI catalysts before and after Mo loading.
131 191 77 64 61 23 Concurrently,
our focus has evolved to study the catalytic activity, selectivity, and coke
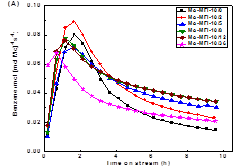
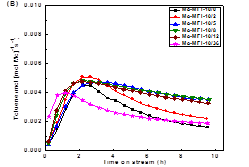
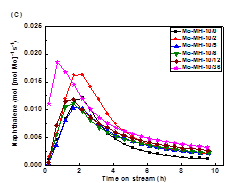
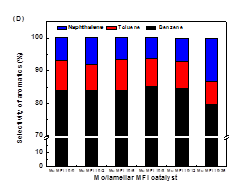
formation on the catalyst in the DMA reactions (Figure 2). For all the
investigated catalyst systems, the aromatic product formation sharply increased
in the initial period of the reaction (i.e., induction period) and reached a
maximum after about 1 h. Afterwards, the production formation rate decreased
with time-on-stream (TOS). The increase in induction time with increasing
zeolite mesoporosity and external surface areas is ascribed to the higher
degree dispersion of Mo species in lamellar MFI and lamellar MWW zeolites,
which is consistent with that indirectly inferred from the Ar isotherm
measurement and DME titrations of Brønsted acidity discussed above. After the reaction
induction period, the methane conversion over Mo/MFI catalysts followed a volcano-shape
plot, consistent with their distribution of the active sites in these catalysts
(Figure 3).
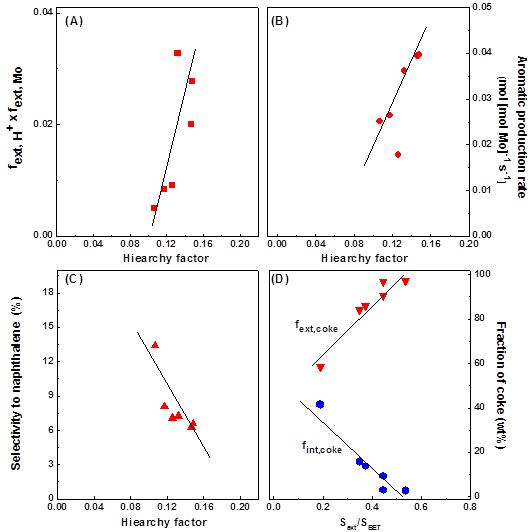
Figure
3. Interdependence between active site distribution, catalytic performance,
and textural properties (described as the product (Vmicro/Vtotal)
x (Sext/SBET)) of the Mo/lamellar MFI catalysts. (A) Active
site distribution, described as the product (fext, Mo) x (fext,
H+), versus hierarchical factor; (B) Aromatic product
formation rate at TOS 10 h versus hierarchical factor; (C) Selectivity
to naphthalene at TOS of 10 h versus hierarchical factor; and (D) Coke
distribution (fext, coke and fint, coke) versus
relative external surface area of the catalysts, respectively.