Reports: UR151369-UR1: Utilizing Halogenated Carbonyls to Direct Stereoselective Synthesis
Todd Davis, PhD, Idaho State University
Former Institution: Idaho State University
Current Institution: U.S. Air Force Academy
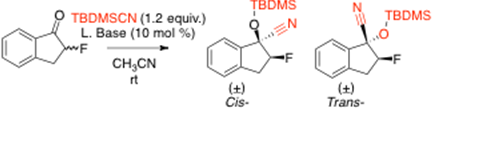
Part 1: Lewis Base Catalyzed TBDMSCN Additions to α-Fluoroketones: Nucleophile Directed Stereochemical Outcome
In the last grant year, our laboratory has focused on substrate scope and optimal reaction conditions for trialkylsilylcyanide (R3SiCN) addition to α-fluorinated ketones. These reactions work well leading to the trialkylsilyl protected cyanohydrin in good yield and selectivity. Our previous work discovered that optimal conditions utilized a nitrogen or phosphorous containing Lewis base in the trimethylsilyl cyanide (TMSCN) additions to α-fluoroketones with reactions going to completion in less than five minutes. With these studies in mind, we investigated the reaction conditions for the tbutlydimethylsilylcyanide (TBDMSCN) addition to α-fluoroindanone. In these transformations, the Lewis base again had a significant effect on reaction time. Investigating a variety of Lewis base catalysts, alkoxides had the shortest reaction time (60 min’s) for the cyanide addition to α-fluoroindanone (Table 1). This contrasts the TMSCN addition as amine or phosphine Lewis base catalysts were favored.
Table 1: Effect of Lewis Base on TBDMSCN Addition to α-Fluoroindanone.
entry |
L. base |
% conversion |
h |
cis:trans |
1 |
CsF |
100 |
24 |
1:8 |
2 |
NMI |
100 |
23 |
1:8 |
3 |
Et3N |
100 |
20 |
1:8 |
4 |
DIPEA |
100 |
19 |
1:8 |
5 |
n-Bu3P |
100 |
20 |
1:8 |
6 |
Ph3P |
41 |
20 |
1:6 |
7 |
iBu3P |
94 |
69 |
1:7 |
8 |
CH3O-+Na |
100 |
1 |
1:9 |
9 |
tBuO_+Na |
100 |
1 |
1:7 |
It is believed that the sterically demanding Lewis base (Table 1, entries 3-5) inhibits the addition of the substrate and therefore slows the reaction rate. However when a less sterically demanding Lewis base is used (i.e. sodium methoxide, Table 1, Entry 8) the addition of the α-fluoroketone is able to activate the carbonyl and subsequent addition of the cyanide nucleophile occurs. The reaction occurs in less than one hour leading to complete conversion and good stereoselectivity (1:9 cis:trans). Based on these studies, the optimal reaction conditions for the addition of TBDMSCN to α-fluoroketones utilizes sodium methoxide as the Lewis base in acetonitrile.
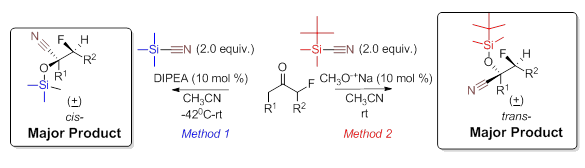
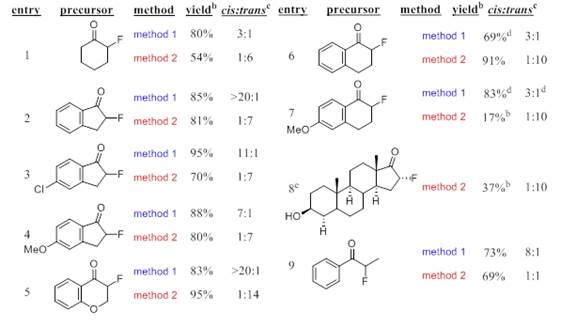
With optimized reaction conditions for both TMSCN and TBDMSCN determined the substrate scope was investigated. Using optimal reaction condition a variety of substrates were examined and in all cases the TMSCN addition led to the cis- while the TBDMSCN yielded the trans- product. As seen in Table 2, these reactions proceed using a variety of cyclic and acyclic α-fluoroketone substrates.
Table 2. Substrate Scope for TMSCN and TBDMSCN Additions
aAll reactions were conducted on a .200M scale based on the starting α-fluoroketone. bYields based on 19F NMR using o-fluorotoluene as the internal standard. cCis/trans ratio were determined using 19F NMR. dFrom reference. eReaction was conducted in a CH3CN/DMF (2.3:1) solvent mixture due to the low solubility of starting material in CH3CN.
The methods tolerate both electron withdrawing and donating groups attached to the aromatic ring however longer reaction times are required when strong activating moieties are used (Table 2, entries 3,4, & 7). The stereochemical outcome predicts that two different reaction mechanisms are under operation. Currently, we are examining low temperature NMR studies to investigate the coordination Lewis base and organosilane (Lewis acid) interaction as well as between the substrate and the Lewis acid.
Part #2: Lewis Base Catalyzed Cyanide Additions to α-Chloroketones
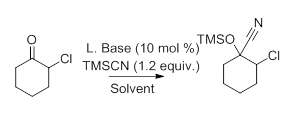
Based on our promising results in the Lewis base addition of trialkylsilane addition to α-fluoroketones we next looked at α-chloroketones. Due to the decrease in electronegativity of chlorine, and the weaker bond dissociation of silicon-chlorine, it is expected that these reactions would be slower in comparison. Initially, using n-Bu3P as the Lewis base, the reaction of TMSCN in a variety of solvents was investigated. The reaction worked well in acetonitrile and DMF going to completion in 24 hours. Although CH3CN and DMF worked the best the diasteroselectivity is poor (2:1).
Table 3: Investigation of Lewis base and Solvent on TMSCN Addition to α-Chlorocyclohexanone.
Entry |
Solvent |
L. Base |
Time (h) |
% Conversion |
dr |
1 |
Toluene |
<span' italic';"="Italic';"">n-Bu3P |
24 |
33 |
1:1 |
2 |
CH3CN |
n-Bu3P |
24 |
100 |
2:1 |
3 |
DMF |
n-Bu3P |
24 |
100 |
2:1 |
4 |
THF |
n-Bu3P |
24 |
5 |
8:1 |
5 |
CH2Cl2 |
n-Bu3P |
24 |
22 |
2:1 |
6 |
CH3CN |
CsF |
24 |
100 |
2:1 |
7 |
CH3CN |
Ph3PO |
24 |
100 |
2:1 |
8 |
CH3CN |
Et3N |
24 |
100 |
2:1 |
9 |
CH3CN |
DIPEA |
24 |
100 |
2:1 |
Next, the role of the Lewis base was examined. In 24 hours, all reactions proceeded to completion with the exception of triphenylphoshine oxide. Although all of the reactions went to completion the diastereoselectivity is poor. To address this problem, we will investigate temperature, order of additon, and the use of a syringe pump to hopefully increase the diastereoselectivity in the addition reactions. Once these reactions are complete we will investigate the derivitization of these molecules to epoxides and azetidines.
Undergraduate Involvement:
Over the course of the 2013-2014 this PRF grant directly supported three undergraduate researchers. One student was funded over the summer and two worked a total of 14 hours per week during the academic year. One student presented her research at the 2013 American Chemical Society Meeting in Indianapolis, IN.
Presentations:
As noted above one undergraduate presented a poster at the Fall ACS meeting and the PI delivered two invited presentations based on the research funded by the ACS-PRF. The first was invited at the National ACS Meeting in Indianapolis, IN during an undergraduate symposium focusing on cutting edge research being conducted at predominately undergraduate Universities (PUI’s). The symposium was entitled: "Small Splash Big Waves: Research at Predominately Undergraduate Institutions." The second talk was delivered at the U.S. Air Force Academy and entitled: "Adventures in an Undergraduate Research Lab".