Reports: UNI753097-UNI7: Structure-Activity Study of Triazolium-Containing Michael Addition Polyesters
Kevin M. Miller, PhD, Murray State University
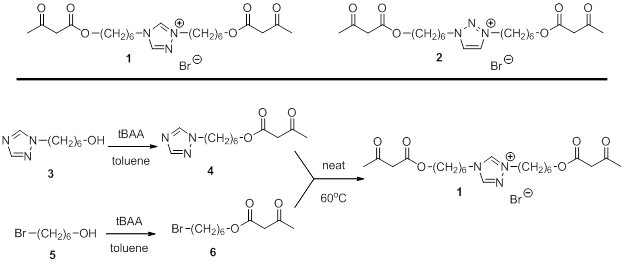
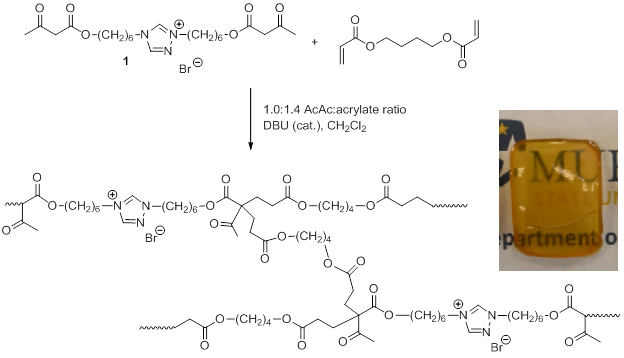
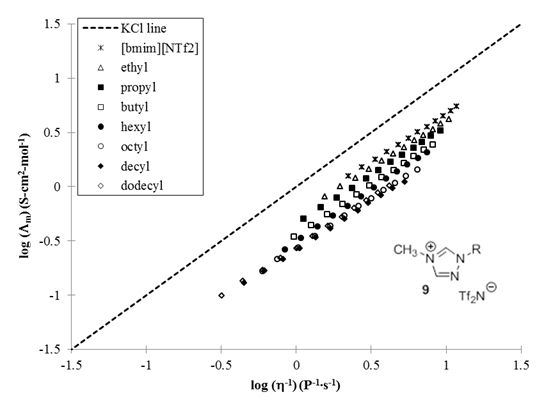
Kevin M. Miller, PhD, Murray State University



Copyright © American Chemical Society