Reports: DNI752705-DNI7: Novel Polymeric Architectures from Monomers with Multiple Reactive Centers
Qianli Rick Chu, PhD, University of North Dakota
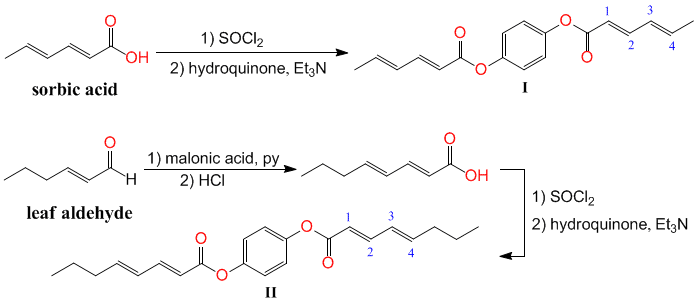
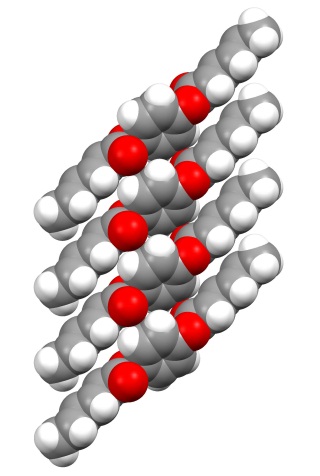
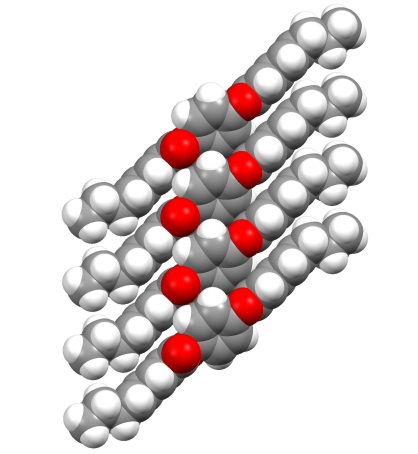
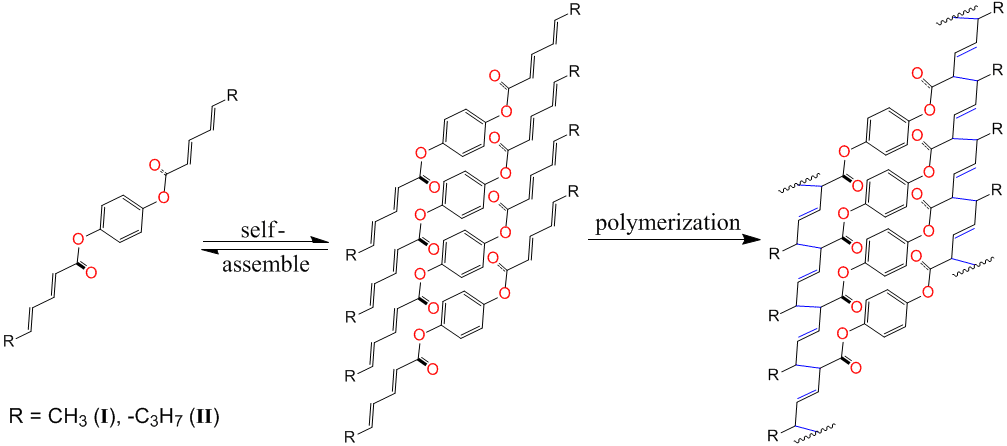
Qianli Rick Chu, PhD, University of North Dakota




Copyright © American Chemical Society