Reports: DNI1052834-DNI10: Identification of Lithium Ion Battery Electrode Structural Inhomogeneity and Its Effect on Battery Performance
Likun Zhu, PhD, Indiana University-Purdue University, Indianapolis
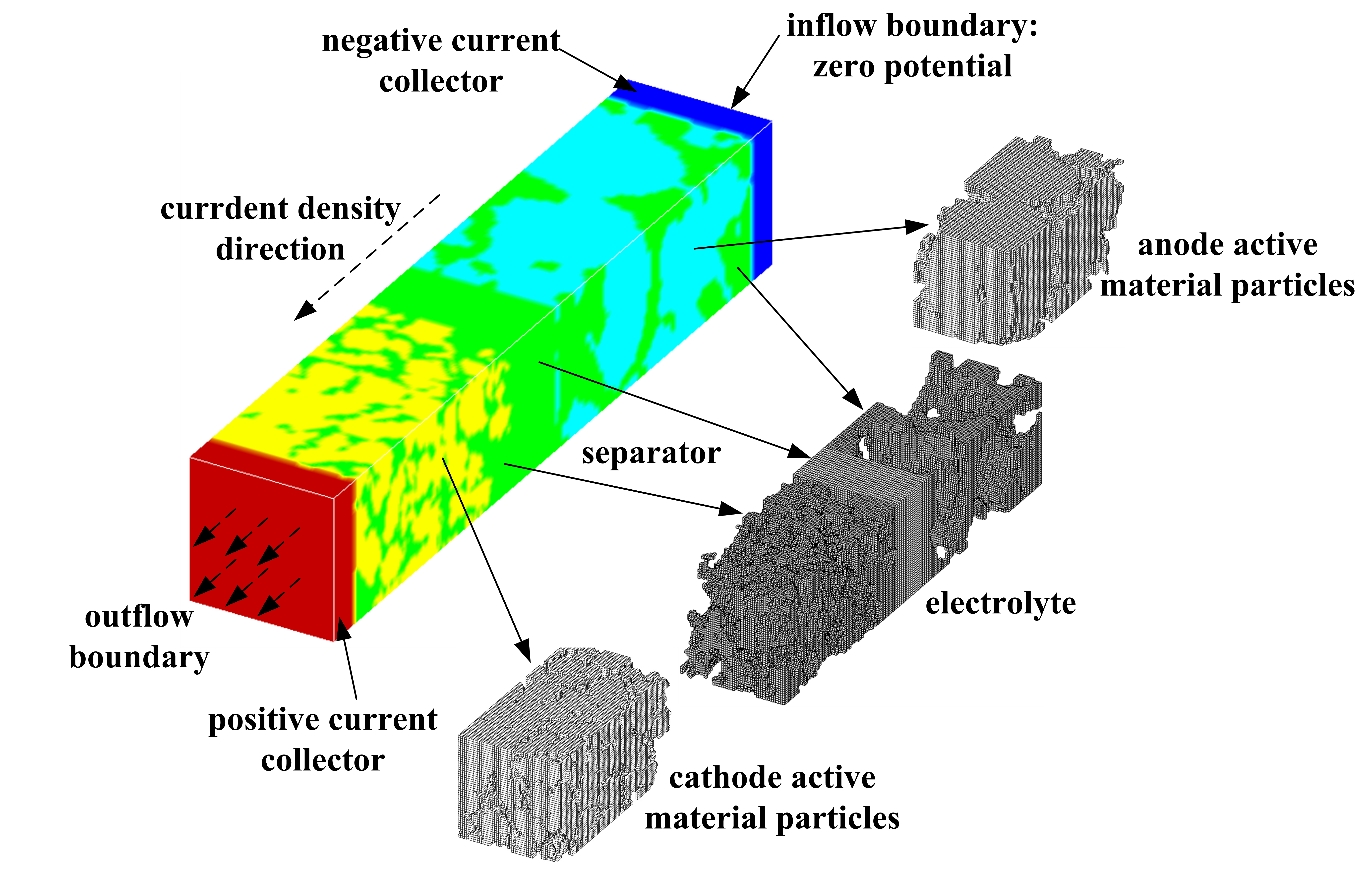
Fig.2 Schematic of a testing
cell including anode, cathode, separator, and current collectors.
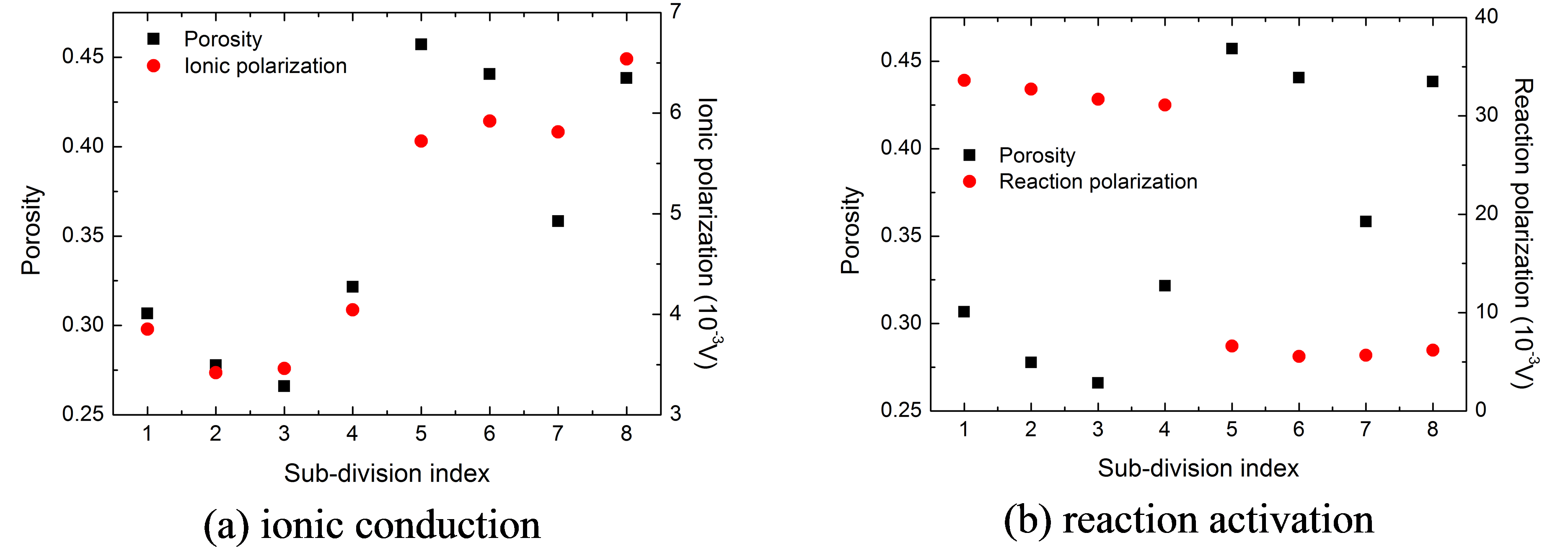
Fig.4 Polarization of each sub-division due to ionic
conduction and intercalation reaction at 300 s during 10 C discharge process at 25