Reports: UNI553617-UNI5: Engineering of Interfacial Barrier Properties to Reduce Oxidation in Emulsions
Rohan V. Tikekar, Drexel University
Synthesis of Si-ε-polylysine (EPL) hybrid microparticles: Eight percent (w/v) LUDOX®HS-30 (anionic) colloidal silica solution and 8% EPL (cationic) solution were prepared in ultrapure water. To prepare EPL coated silica hybrid microparticles, the anionic silica nanoparticle solution and cationic EPL solution were mixed in 1:1 volume proportion. The total volume of the aqueous phase was 25mL. The solution was stirred for 30 minutes to facilitate hybrid microparticles formation. The pH of aqueous phase was adjusted to 6. 4% LUDOX®HS-30 solution adjusted to pH of 6 was also prepared to synthesize Si colloidosomes.
Synthesis of colloidosomes:
Characterization of colloidosomes:
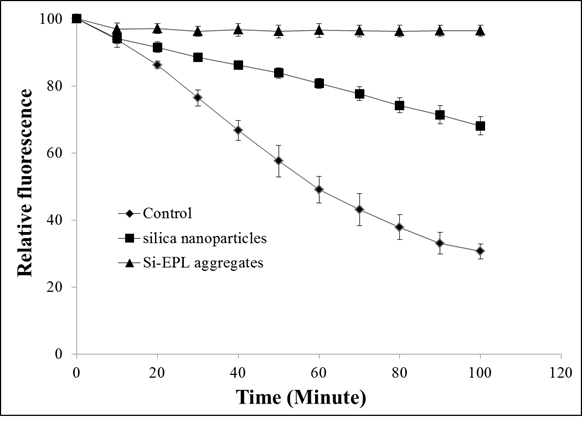
Measurement of barrier
properties of Colloidosomes: where, It AAPH is
the fluorescence intensity of the sample after ‘t’ minutes upon exposure to
AAPH, I0 AAPH is the fluorescence intensity of the
sample immediately after adding AAPH, It control is the
fluorescence intensity of control after ‘t’ minutes, I0 control
is the fluorescence intensity of control immediately after adding AAPH.
RESULTS AND DISCUSSION Characterization of Si-EPL hybrid
microparticles and Colloidosomes: The average particle size of silica
nanoparticles and Si-EPL hybrid microparticles was 11.6±0.56 nm and 414±44.4 nm
respectively. The SEM image of Si-EPL hybrid microparticles is shown in Figure
1. Large non-uniform particles formed due to aggregation of anionic silica
and cationic EPL could be observed clearly. The average particle size obtained
based on the image analysis of 19 hybrid microparticles was approximately
339±63 nm. Thus the average particle sizes measured by DLS and SEM were
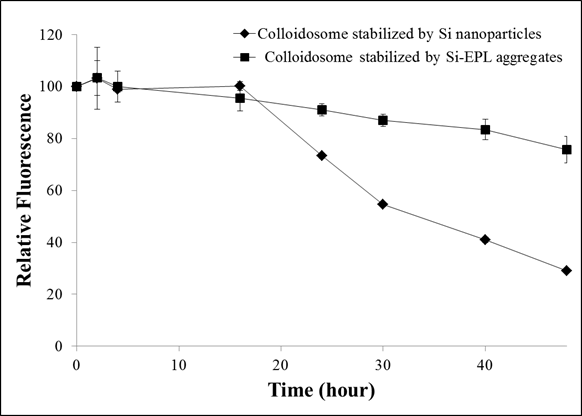
approximately similar. The average droplet sizes colloidosomes stabilized by
silica nanoparticles and silica-EPL microparticles were 118±55.3 nm and 679.5±47.5 nm respectively. These results
demonstrate that formation of polymer silica hybrid complex significantly (p<0.05)
increased the size of the silica particles and the resulting colloidosomes.
Figure 1: Figure 2:
![]()