Reports: UR252201-UR2: Speciation and Sequestration of Rhenium in Sulfidic and Polysulfidic Natural Waters
Trent Vorlicek, PhD, Minnesota State University Mankato
Project Objectives: The overarching purpose of this project is to define better the chemical pathway leading to Re deposition within anoxic waters. Recent evidence from the Black Sea suggests that Re removal may be linked to Mo deposition via co-precipitation of Re with a posited FeMoS solid phase (i.e., Fe5Mo3S14). This potential relationship has led the project to include batch experiments involving formation of FeMoS solid(s) in the presence of trace concentrations of ReO4-. These experiments aim to determine thermodynamic constants for the FeMoS solid(s) as well as quantify any fractionation occurring between Re and Mo during their possible co-precipitation. The project also seeks to develop a reverse phase ion pair chromatography (RP-IPC) method for separating mixtures of all oxythiomolybdates (MoO42-, MoO3S2-, MoO2S22-, MoOS32-, and MoS42-) and all oxythioperrhenates (ReO4-, ReO3S-, and ReS4-; ReO2S2- and ReOS3- are kinetically unstable.). Presently, Mo and Re speciation can only be predicted from thermodynamic data. Eventual coupling of RP-IPC to ICP-MS may allow for quantifying actual Mo and Re speciation within natural sulfidic waters. Because Mo and Re have stable isotopes, RP-IPC with MS may be used in the future to quantify isotopic fractionation occurring as MoO42- or ReO4- transforms into MoS42- or ReS4-.
Research Progress:
a.) Co-precipitation of Re with an FeMoS solid phase
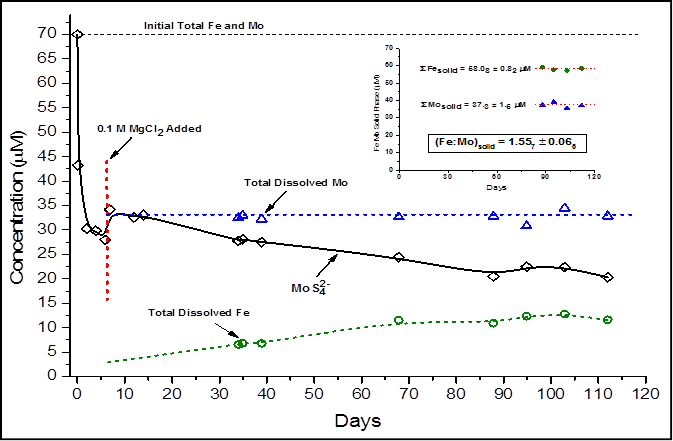
Figure 1 shows the reaction progress upon addition of 70 The inset in the figure shows data related to the FeMoS
solid phase. The y-axis in the inset is calculated from the difference between
initial total Mo and Fe and equilibrium values of dissolved Mo and Fe. Total
Fe and total Mo associated with the FeMoS solid yield an Fe:Mo ratio of 1.56 ±
0.07. This ratio is in very good agreement with that of Fe5Mo3S14
(Fe:Mo = 1.7 ± 0.7), the solid posited to account for Mo removal (and possibly
Re) in euxinic water columns.
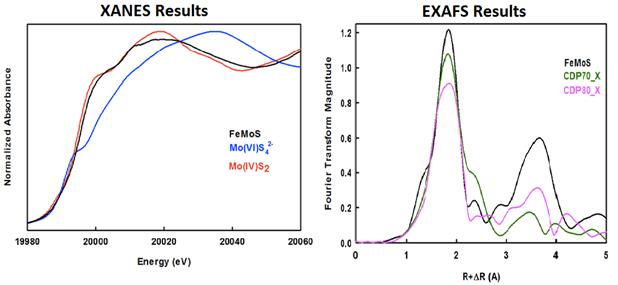
The solid formed after 112 days in the solution described in
Fig. 1 was isolated under an N2 atmosphere and subjected to X-ray
absorbance spectroscopic (XAS) analyses at the Advanced Photon Source associated
with Argonne National Laboratory. The XAS work was performed by Dr. Anthony
Chappaz with whom I collaborate on research related to trace metal
geochemistry. Figure 2 shows XANES and EXAFS spectra of the FeMoS solid as well
as those of various standards. XANES results suggest that the FeMoS solid
contains MoIV. Modeled interatomic distances for the FeMoS solid from
EXAFS spectra are consistent with those of a FeMoS cubane cluster. Most
intriguing, the EXAFS spectra of the synthesized FeMoS solid agree with major
aspects of spectra previously taken of the form of Mo found within 7000 to 8000
year old Lake Cadagno sediments (black vs. green or pink spectra). Taken
together, these data suggest that we have reproduced in the lab a naturally
occurring pathway to Mo burial. At present, Re:Mo ratios within the synthesized
FeMoS solid and equilibrated aqueous phase have not been quantified for characterizing
any Re and Mo fractionation during possible co-precipitation. However, several
batch experiments over a range of solution conditions await ICP-MS analyses
over the coming year.
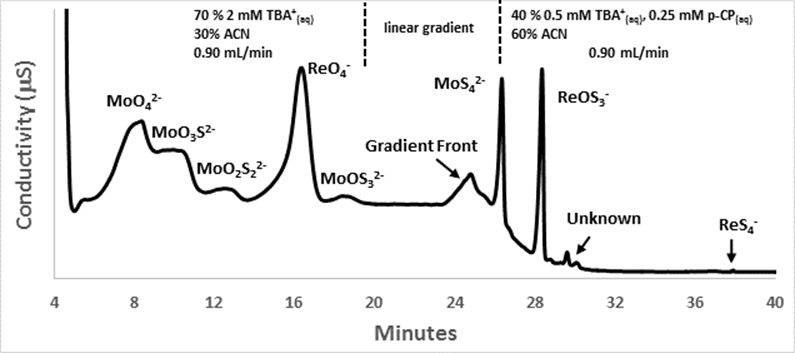
b.) Separation of all
oxythiomolybdate and all oxythioperrhenate anions Figure 3 displays a chromatogram demonstrating the successful
separation of a mixture containing all eight oxythiomolybdate and
oxythioperrhenate anions at Student participation, publications, and presentations: One
publication in Metallomics and three presentations were supported by PRF
during 2013-2014. A total of five undergraduates participated in the project.
Two students presented their results at the Spring 2014 ACS national meeting.
The PI presented results at the Fall 2013 ACS national meeting as well as the
Goldschmidt Conference in Spring 2014.