Reports: ND751827-ND7: Photolabile Protecting Group-Based New Method for the Development of Photodegradable Polymers
Pengfei Wang, University of Alabama at Birmingham
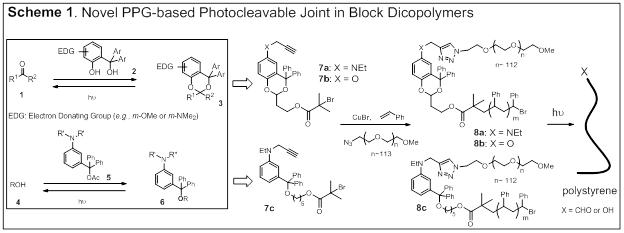
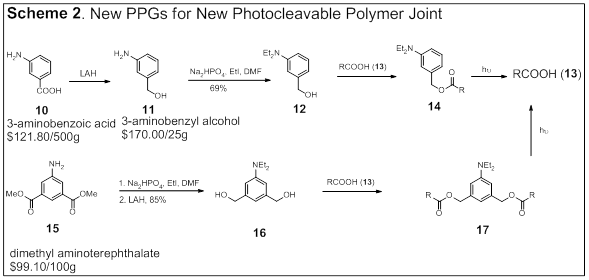
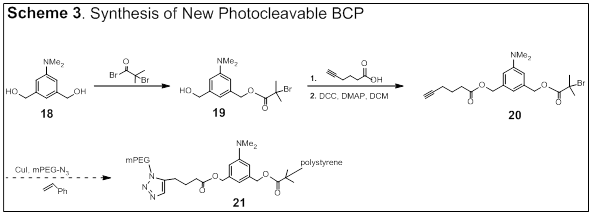
Pengfei Wang, University of Alabama at Birmingham



Copyright © American Chemical Society