Reports: DNI651672-DNI6: The Role of the Support in MAO (Methylaluminoxane) Activated Olefin Polymerization
Eva Zurek, PhD, State University of New York at Buffalo
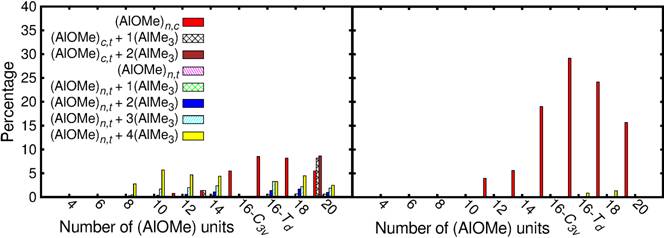
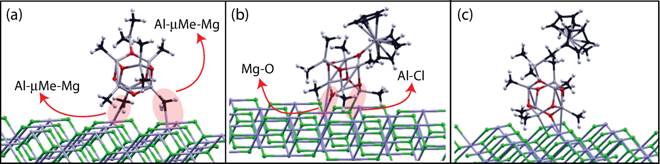
Eva Zurek, PhD, State University of New York at Buffalo


Copyright © American Chemical Society