Reports: UR453166-UR4: Solid-State Chemistry of the Nitrile Oxides
William Hubert Ojala, University of St. Thomas
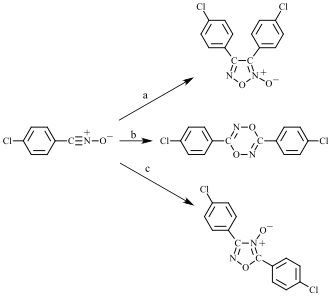
Figure 1. Dimerization of a nitrile oxide (shown here for 4-chlorobenzonitrile oxide) in solution can yield a furoxan (top), dioxadiazine (middle), or oxadiazole-4-oxide (bottom), depending on the reaction conditions: a = inert solvent; b = pyridine-catalyzed; c = trimethylamine-catalyzed
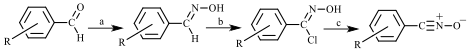
In this first year of our grant, we began preparing a variety of nitrile oxides with the immediate goal of determining their crystal structures by single-crystal X-ray diffraction. We followed a standard method for synthesizing our compounds, a method that involves chlorination of the substituted benzaldoxime followed by dehydrohalogenation in base (Figure 2). We attempted the synthesis and isolation of 4-bromobenzonitrile oxide, 4-chlorobenzonitrile oxide, 3-bromobenzonitrile oxide, 4-nitrobenzonitrile oxide, 3-nitrobenzonitrile oxide, 4-fluorobenzonitrile oxide, and piperonal nitrile oxide. Crystallization of the nitrile oxides was complicated by their tendency to dimerize as well as by their relatively high solubility in the crystallization solvents chosen (usually carbon tetrachloride or petroleum ether).
Figure 2. Typical synthetic route to a benzonitrile oxide from a benzaldehyde derivative;
a = hydroxylamine hydrochloride, base; b = N-chlorosuccinimide; c = aqueous base.
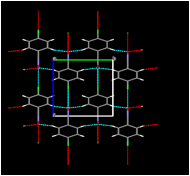
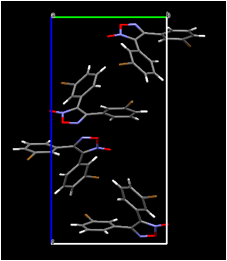
In several cases, the infrared spectra of our unrecrystallized products showed a band at approximately 2250 cm-1 confirming the presence of the carbon-nitrogen triple bond. At this time, we have determined the crystal structures of two compounds prepared in this study: 4-chlorobenzonitrile oxide and bis(3-bromophenyl)furoxan. In work previous to the current investigation, we determined that the product of the solid-state dimerization of 4-chlorobenzonitrile oxide is the furoxan, the "head-to-head" dimer; we also determined that of the two known polymorphs of this furoxan, it is the monoclinic polymorph and not the orthorhombic polymorph that is formed in the crystal. In light of the fact that the "head-to-head" dimer is formed in the solid state as well as in inert solvents, it is of interest that calculations (Yu, Z.-X.; Caramella, P.; Houk, K. N., J. Am. Chem. Soc. 2003, 125, 15420-15425) indicate that the transition state of the dimerization is centrosymmetric. Our examination of the molecular packing arrangement of 4-chlorobenzonitrile oxide (Figure 3) thus far has shown that the closest intermolecular approach (3.865 in Figure 3) is between translationally related molecules, which is consistent with head-to-head dimerization; on the other hand, the intermolecular approaches do not indicate that other dimerization modes are impossible. A further significant feature is the close oxygen-chlorine intermolecular approach of 3.1683(16) between neighboring molecules. If this halogen-bonding interaction occurs in other nitrile oxides, changing the position of the halogen on the ring is expected to dramatically affect the packing arrangement, possibly changing it to one conducive to the formation of the head-to-tail dimers rather than the head-to-head dimer. We are continuing to pursue this possibility in our laboratory.
Figure 3. (Left) View of molecular packing in 4-chlorobenzonitrile oxide along (100) showing contacts shorter than the sum of the van der Waals radii as dotted lines; (Right) view of neighboring molecules and some intermolecular approaches relevant to solid-state dimerization (distances in angstroms).
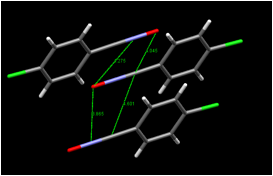
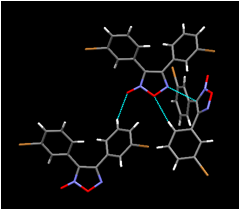
Our investigation of the solid-state chemistry of nitrile oxides extends to the crystal structures of their dimers as we examine both kinds of structures for features that might strongly influence their molecular packing. In our crystal structure of bis(3-bromophenyl)furoxan (Figure 4), we do not observe a close (less than the sum of the van der Waals radii) oxygen-bromine contact corresponding to the close oxygen-chlorine contact found in our nitrile oxide structure. Halogen atoms in our furoxan structure instead appear to play a space-filling role, in contrast to the more directional electronic role they play in our nitrile oxide structure. The influence, or lack of it, exerted by halogen bonding in these structures is subtle and thus is of ongoing interest to us. Attempts to grow X-ray quality crystals of our other nitrile oxides and the corresponding dimers we have prepared thus far will continue into the next year of this project.
Figure 4. (Left) View of the molecular packing in bis(3-bromophenyl)furoxan along (100); (Right) view of neighboring molecules showing (as dotted lines) contacts shorter than the sum of the van der Waals radii involving the ring heteroatoms.
Although our project is still in its early stages, it is clear that it is of direct relevance to the rapidly developing field of crystal engineering. It is our intention to eventually publish our results, perhaps in the ACS journal most directly relevant to this field, Crystal Growth and Design. The three students in our group supported by ACS-PRF during Summer 2014 intend to participate in the CHED undergraduate poster session at the March 2015 ACS National Meeting in Denver, Colorado. For them, this work has provided research experience that will advance their careers in the sciences, and it is my hope that they will find the organic solid state in particular to be a compelling area for their own future investigation.