Reports: UR552049-UR5: Synthesis and Multilayering of Poly(4-vinylphenol) Derivatives for Future Applications as Proton Exchange Membranes in Fuel Cells
Ronny Priefer, PhD, Western New England University
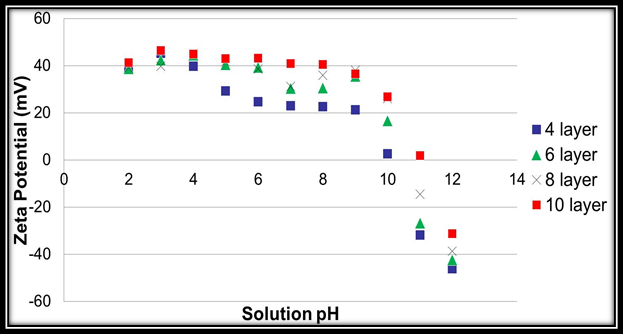
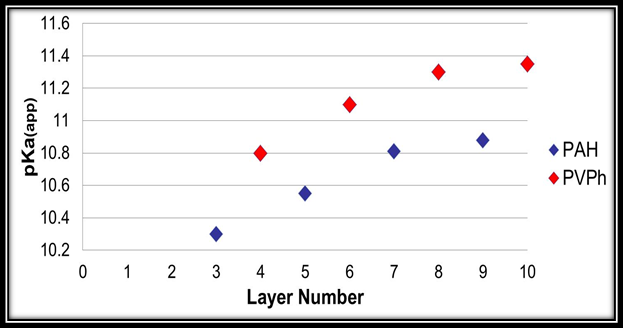
Firstly, during our first year of work on this project we successfully synthesized poly(4-vinylbenzeneboronic acid). We spent the majority of this past year multilayering this novel pseudo-polyelectrolyte with poly(allylamine hydrochloride) onto quartz surfaces. We were able to perform this task and are only a few AFM thickness measurements away from submitting this work for possible publication. In addition, we felt that an important hypothesis of the grant is that having matching pKa’s of the two multilayered polymers is a key factor that has produced our strong conductivity results. However, it has been well established that once polymers are incorporated into multilayer systems their pKa’s will change to either a higher or lower value. This has been determined by multilayering polymers with varying layer numbers onto silica colloids and determining their zeta potentials. Since we have pioneered the work on multilayering pseudo-polyelectrolytes our particular systems have never been evaluated. Thus, using 70nm Snowtex silica particles obtained from Nissan Chemical Industries we successfully multilayered poly(allylamine hydrochloride) with poly(4-vinylphenol) as well as the aforementioned poly(4-vinylbenzeneboronic acid) with poly(allylamine hydrochloride). We subsequently have just completed the data collection of their corresponding zeta potentials at different assembly pH’s, layer numbers, and solution pH’s using our Beckman Coulter’s Delsa Nano C Particle Analyzer. We have completed the data analysis of this and we can state that the pKa’s of poly(allylamine hydrochloride), poly(4-vinylphenol), and poly(4-vinylbenzeneboronic acid) are indeed very close when multilayered at six total layers and higher (Figures 1 and 2). This validates our initial hypothesis. This work is currently being composed for submission but was presented at this year’s American Chemical Society National Meeting in San Francisco as well as with students at the Eastern Colleges Science Conference (ECSC) at Marist College in Poughkeepsie, NY.
Figure 1. Acid-base equilibrium profile for 4, 6, 8, and 10-layer PAH/PVPh film assembled at pH 11.5.
Figure 2. Dependence of the apparent dissociation constant of PAH and PVPh on the total number of layers in PAH/PVPh multilayer films assembled at pH = 11.5.
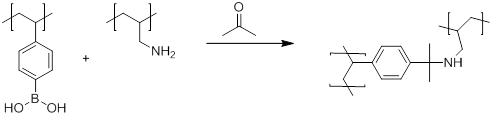
As a side, we stumbled upon the crosslinking reaction of poly(4-vinylbenzeneboronic acid) and poly(allylamine hydrochloride) with acetone once absorbed onto surfaces. This led to a branched off area of research of using this novel system as a breathalyzer for monitoring diabetic individuals. We had much international media attention on this which we have submitted a patent as well as an American Diabetes Association grant proposal which is currently under review.
Figure 3: Proposed Petasis reaction to cross-link PVBBA with PAH
Secondly, we have been able to synthesize some of the polymers as delineated within the grant, however a derivative of poly(4-vinylphenol) that we had always believe inaccessible was the 2-acetyl compound. This was initially believed to be not possible since our synthetic route utilizes a Wittig reaction. We however have been able to successfully synthesize this polymer via a photo-Fries rearrangement of poly(4-acetoxystyrene). We have also been able to multilayer this new pseudo-polyelectrolyte with poly(allylamine hydrochloride) onto both quartz slides and Snowtex silica particles. We still need to perform some analysis (in particular AFM) prior to submission for publication.
Thirdly, through collaborations with Drs. Jennifer Mallory and Anthony Santamaria from the College of Engineering here at Western New England University we have been working on various different approaches towards developing these thin films to act as proton exchange membranes. These include “Design of a Spin Coating System for Thin Films in Proton Exchange Membrane (PEM) Applications” and “Atomization and Sprays: Design and Fabrication of an Impinging Jet System for the Development of Proton Exchange Membranes” which were senior engineering projects and were presented at local conferences.
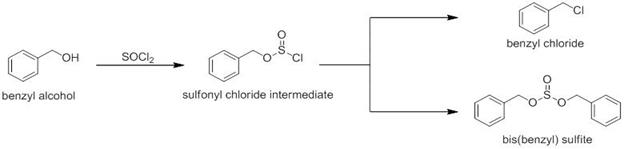
Finally, in our initial grant proposal we had indicated that the route that would provide the styrene derivatives would come from the reaction of a benzaldehyde with a Wittig reagent. We felt that in certain instances this may be problematic; hence we thought that we could reverse this system, whereby we would convert our aryl moiety into the Wittig reagent and add it to formaldehyde. Thus, starting from a benzyl alcohol we envisioned conversion to the benzyl chloride initially with simply thionyl chloride. This reaction did not always provide the expected product, but instead occasionally yielded the dibenzyl sulfite (Figure 4). We therefore further examined this phenomenon and ultimately published the results in Tetrahedron Letters earlier this year.
Figure 4: Chlorination and sulfite formation from the reaction of thionyl chloride and benzyl alcohol