Reports: UR453159-UR4: Impact of Solvent-Solute Interactions on Photochemistry of p-Aminobenzoic Acid Derivatives
Sarah J. Schmidtke Sobeck, PhD, College of Wooster
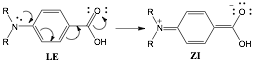
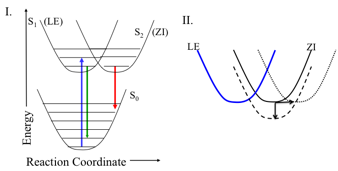
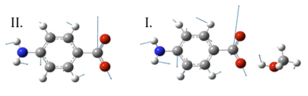
Sarah J. Schmidtke Sobeck, PhD, College of Wooster



Copyright © American Chemical Society