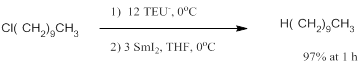Reports: UR152067-UR1: The Characterization and Synthetic Utility of Complexes Made from Samarium Diiodide and Anionic Phosphoramides
Chriss E. McDonald, PhD, Lycoming College
Samarium diiodide is one of the most useful reagents employed by organic chemists. The utility of this reagent is greatly enhanced by the addition of electron rich cosolvents. HMPA (1, Scheme 1) is the most common cosolvent to be employed and the majority of modern SmI2 chemistry is accomplished with this cosolvent (Scheme 1). Unfortunately HMPA is a mutagen which has leaded to an extended search for suitable alternatives. The goals of this work are to find SmI2 activators which are both safer and more effective. We have recently reported that TPPA (2) is approximately an order of magnitude better at activating SmI2 at two of its key synthetic processes, reduction of carbon-halogen bonds and reduction of ketones. Our most recent published work involves the use of deprotonatable phosphoramides such as DPMPA (3). Complexes formed from SmI2 and four equivalents of the anion derived from deprotonation of 3 (DPMPA-) proved to be > 100X more reactive than the SmI2/HMPA complex as determined by the reduction of 1-chlorodecane.
Scheme 1. Phosphoramides
Recently we have focused on extending this approach to ureas. Advantages of ureas over phosphoramides include lack of mutagenicity, lower molecular weight, and ease of synthesis. The most common urea used in synthesis, DMPU, is known to be a modest activator for SmI2. Initially we constructed a series of deprotonatable ureas (Scheme 2) and assayed them with regard to their ability to activate SmI2 for the reduction of 1-bromodecane and the less reactive 1-chlorodecane.
Scheme 2. Ureas
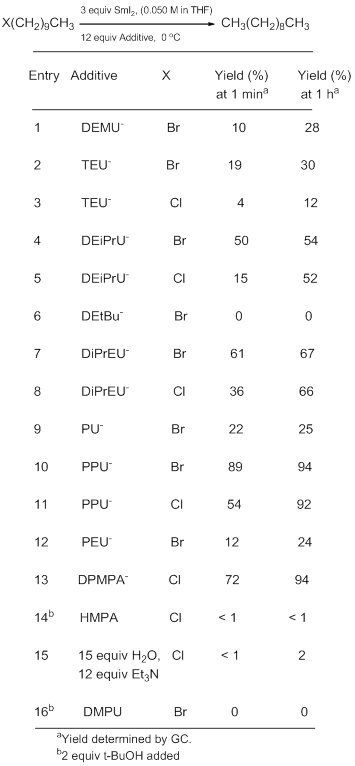
In contrast to our earlier work with DPMPA, it was decided to eliminate a proton source from the reaction mixture because of the ambiguity induced to the ligand (neutral vs. anionic). As can be seen in Table 1, SmI2/anionic urea complexes are substantially more reactive than other synthetically useful SmI2 complexes involving HMPA, H2O/Et3N, and DMPU. They are not as reactive as SmI2/DPMPA-, but SmI2/PPU- approaches that level of reactivity.
Table1. The Reduction of 1-Halodecanes with SmI2 and Additives
As expected it was substantially more difficult to reduce 1-chlorodecane. Because of its low molecular weight and ease of synthesis and purification, TEU (triethylurea) was selected for further study. PPU, based on its greater reactivity was also selected. We were intrigued to note that for SmI2/TEU-, the order of addition matters a great deal. The results in Table 1 were generated in experiments where the 1-halodecane was added last. In experiments where the SmI2 is added last, the yields of decane increase substantially. This order of addition phenomena is repeatable but not well understood.
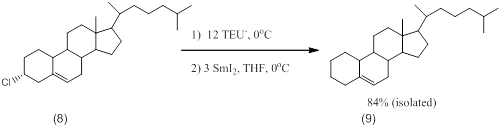
We have used this procedure to reduce a variety of alkyl halides. The reduction of cholysteryl chloride is illustrative.
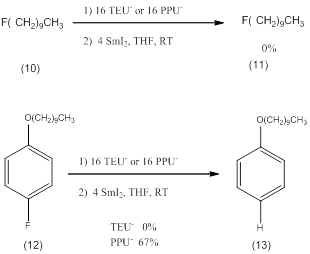
It is possible to reduce some carbon fluorine bonds. Aryl fluorides can be reduced with SmI2/PPU- but not the less reactive SmI2/TEU-.
We have used SmI2/2 TEU- and SmI2/4 TEU- to reductively cyclize chloro and bromoalkenes as shown in Table 2. To this point our results are slightly inferior to the methodology worked out with SmI2/ 4 DPMPA-. We investigated the effects of changing the ratio of activator to SmI2, as well as the starting temperature. As in the simpler reductions, SmI2 is added last in these experiment.
Table 2. The Reductive Cyclization of Haloalkenyl Naphthalenes.
Current work in this laboratory focuses on the use of other reluctant radical precursors, as well as the development of new SmI2 reactions.