Reports: ND352856-ND3: Stabilization of Low-Oxidation State Main Group Complexes with sigma-Acceptor Z-Type Ligands
Rudolf J. Wehmschulte, Florida Institute of Technology
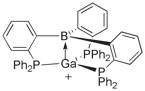
Figure 1. Sketch of a target compound.
Ligand Synthesis
We have prepared the ligand B(C6H4PPh2)3, and the more soluble isopropyl substituted analogue B(C6H4PiPr2)3, is currently being synthesized- We have also prepared the monodentate FLP type ligand cis-Mes2P(Ph)C=C(C6F5)B(C6F5)2 (Mes = 2,4,6-Me3C6H2-, FLP = frustrated Lewis Pair), which features an electron rich phosphorus center and a strongly electron poor boron center. The synthesis of mercaptopyridine based ligands such as B(2-S-C5H4N)3 is planned for this semester.
Ga(I) and In(I) Precursors
In(I)triflate. This compound is readily available through the reaction of InCl or In powder with triflic acid in toluene solution. Due to its high solubility in common organic solvents and its surprising resistance to disproportionation it was thought to be a suitable starting material for this project.
Ga+ and In+ Cations. Recently, room temperature stable, arene substituted Ga+ and In+ cations have been obtained through prolonged sonication of arene solutions of Ag[Al{OC(CF3)3}4] with metallic gallium and indium. We have succeeded using a simple thermal process involving Ag[CHB11Cl11] and metallic gallium and indium in toluene, bromobenzene or fluorobenzene solutions.
These compounds are stable in the solid state and aromatic solutions up to at least 80 °C under anaerobic and anhydrous conditions. Two crystal structures were obtained for the indium species. Crystals of the gallium compound so far have been too small for X-ray diffraction studies.
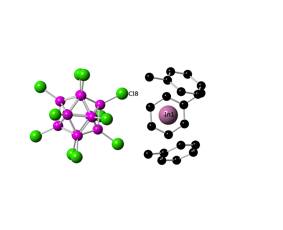
The structure of [In(toluene)3][CHB11Cl11], which was obtained from hot toluene solution, is coordinated by three toluene molecules and loosely by one chlorine center of the anion. This coordination is similar to the one previously reported for [In(C6F5F)3][Al{OC(CF3)3}4].
Figure 2. Crystal structure of [In(toluene)3][CHB11Cl11]
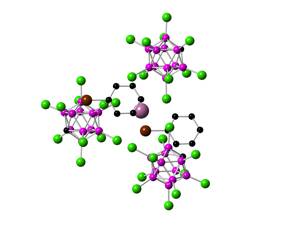
The crystals that were obtained from bromobenzene solution contain the In+ center in four different coordination environments, which is unprecedented to the best of our knowledge. The In+ cation is coordinated to three anions and to one or two solvent molecules in various forms. Interestingly, there is no η6-type coordination to the solvent as in the previous structure, instead an η3-coordination and coordination through the bromine substituent was observed.
Figure 3. Crystal structure of one of the cations in [In(C6H5Br)n][CHB11Cl11]
Reactivity Studies and Complex Formation Solutions of Ga+ and In+ reacted quickly with the ambiphilic ligand B(C6H4PPh2)3 under formation of fine orange solids. Unfortunately, we have not yet been able to identify these solids. We hope that the use of larger amounts or of the more lipophilic ligand B(C6H4Pi We then screened the coordination properties of a number of ligands with respect to In[CB11Cl11]. The phosphines Ph3P and tBu3P did not form any adducts in solution in amounts that could be detected by 31P NMR spectroscopy. The N-heterocyclic carbene IPr ({CHNDipp}2C:, Dipp = 2,6-iPr2C6H3-) and several amines lead to the formation of a fine dark precipitate, most likely indium metal. Crystals obtained from the carbene reaction were identified as the imidazolium salt [(CHNDipp)2CH][CHB11Cl11] by X-ray crystallography.
Solutions of In[CB11Cl11] in fluorobenzene were shown to be inert to alkyl halides such as bromopentane, bromohexene or t-butylchloride, even at 80 °C. We were hoping for an easy entry into the synthesis of a cationic indium(III) species such as [C5H11InBr]+.
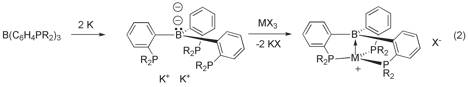
Attempted reduction of B(C6H4PPh2)3 The reaction of a reduced ligand such as [B(C6H4PPh2)3]2- with a metal halide MX3 could be an alternative approach to the target species (eq 2). The radical monoanion [B(C6H4PPh2)3] - or the dianion [B(C6H4PPh2)3]2- would be interesting species in the own regard.
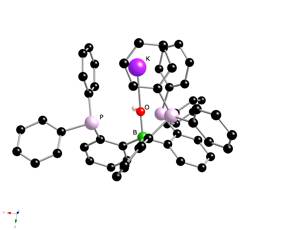
Addition of potassium to a THF solution of B(C6H4PPh2)3 resulted in the slow consumption of potassium (several hours at room temperature) and the formation of a cloudy dark yellow solution. The deep blue or green color expected for the radical anion was not observed. After work-up, a colorless crystalline solid was obtained in approximately 40% yield that was identified as the salt {(Ph2PC6H4)3BOH}Ká2 toluene. Its structure shows that the potassium cation is solvated by the hydroxide oxygen and three phenyl groups from the three Ph2P donor centers. It is interesting that the coordination through the aromatic rings is more favorable than coordination through the phosphine donors. The mechanism of this reaction is not known yet, and more attempts to obtain reduced ligand systems will be tried in the future.
Figure 4. Crystal structure of {(Ph2PC6H4)3BOH}K