Reports: DNI1052827-DNI10: Elucidating the Electronic Structure and Chemistry of Layered Vanadium Oxides for Next-Generation Energy Storage
Louis Piper, PhD, Binghamton University
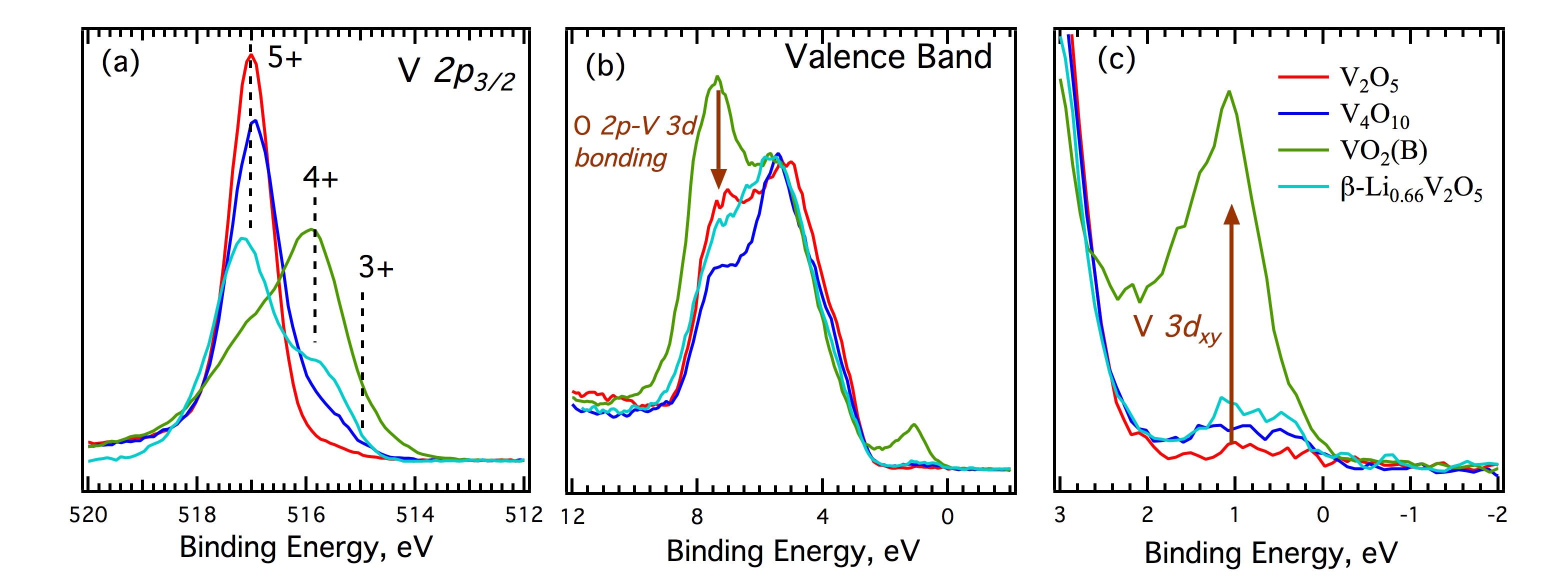
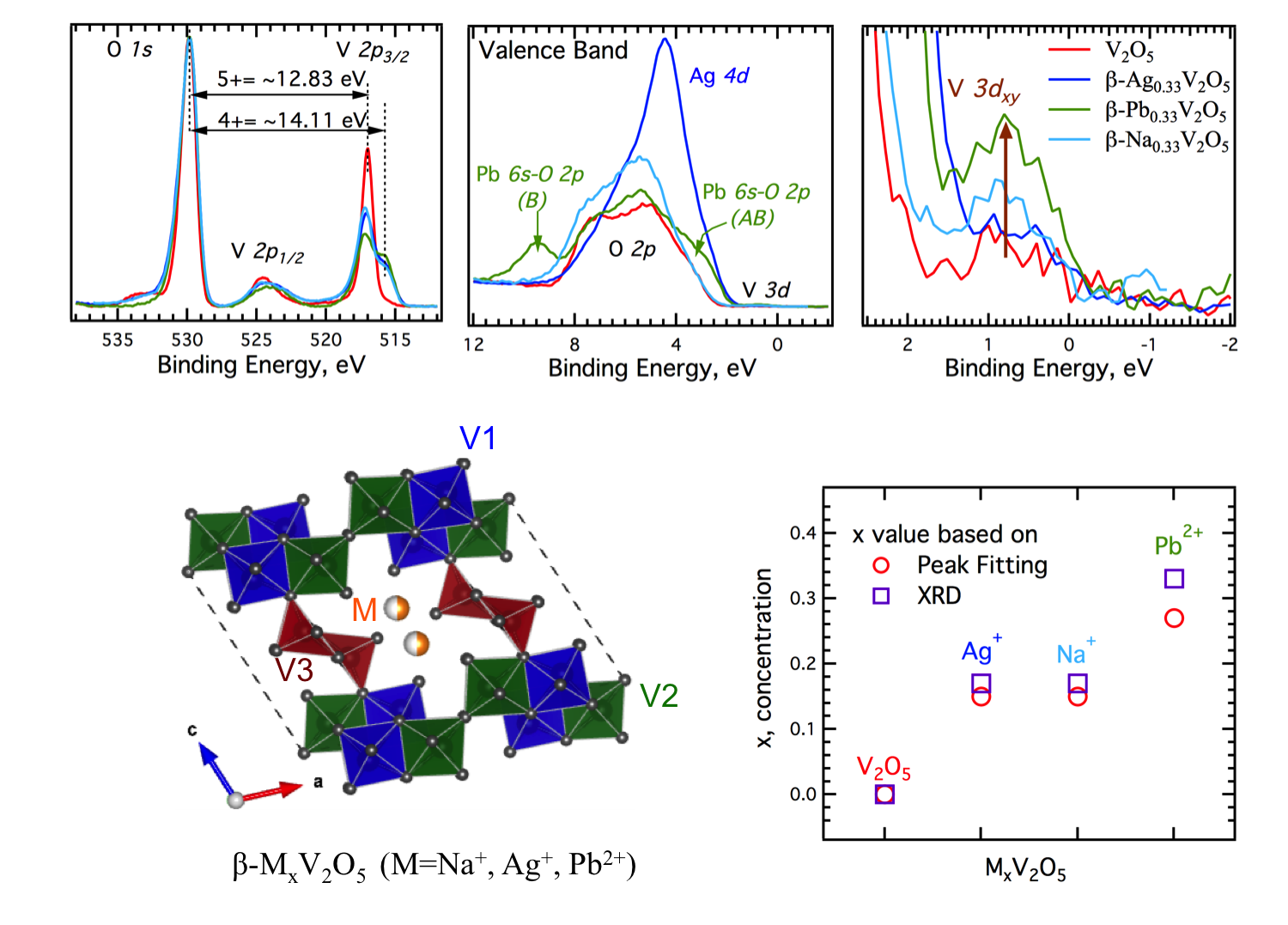
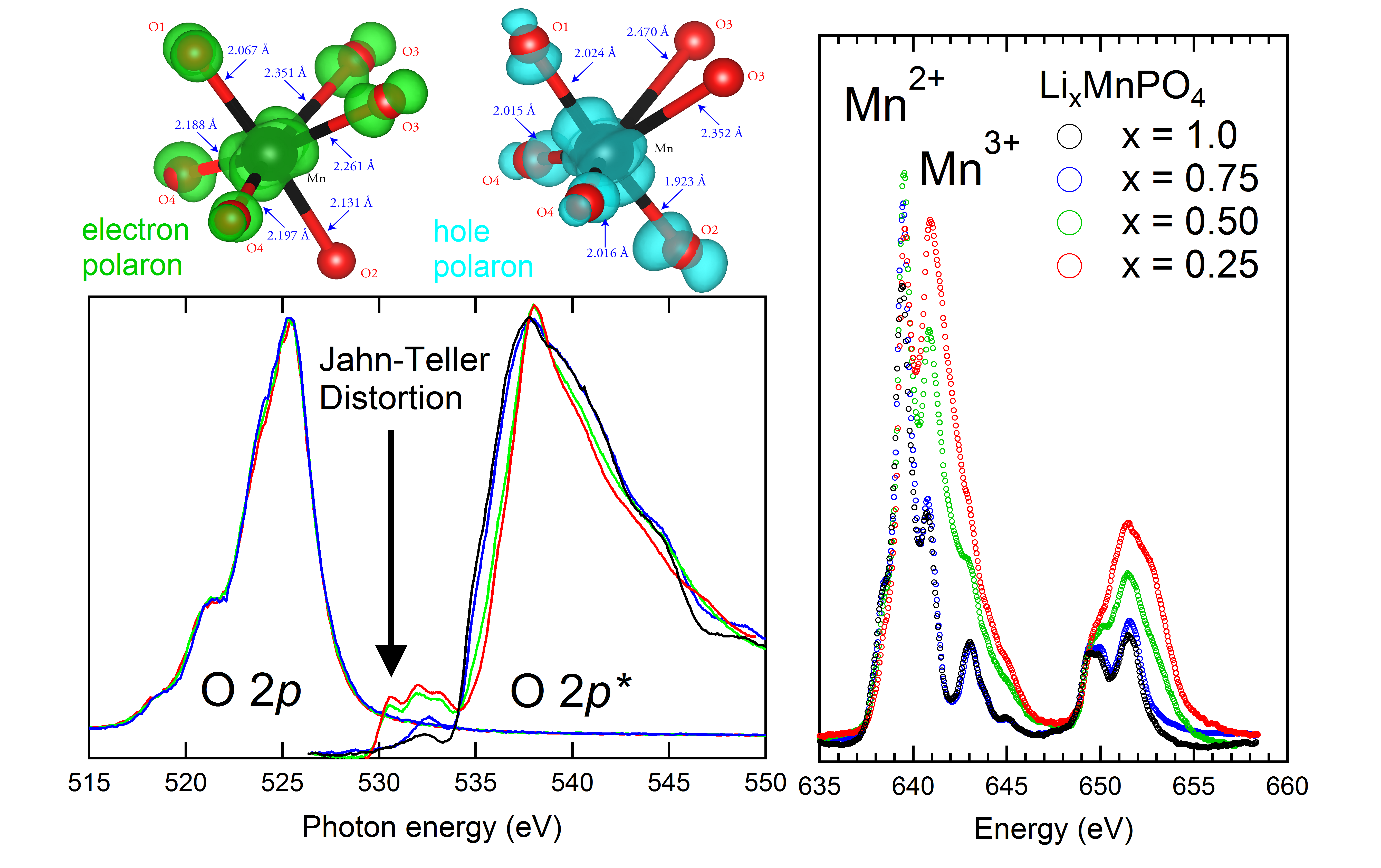
Louis Piper, PhD, Binghamton University



Copyright © American Chemical Society