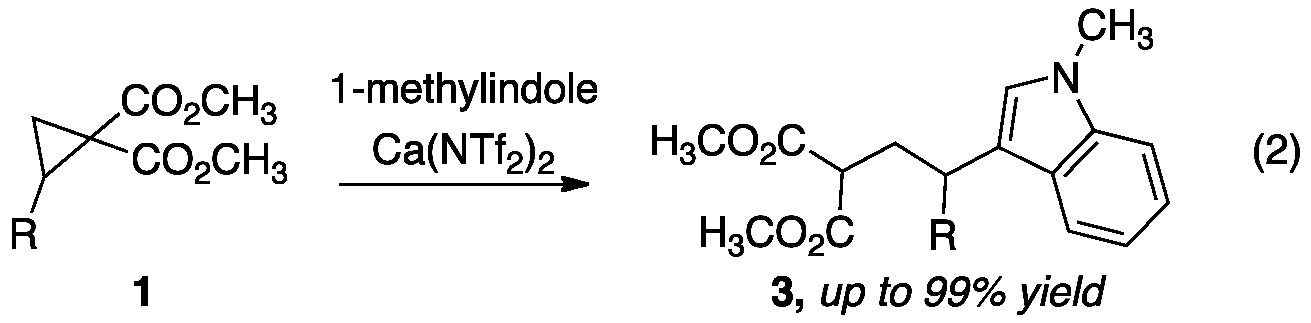Reports: UNI151029-UNI1: Calcium Catalyzed Homologous Conjugate Addition Reactions
Kristine Nolin, PhD, University of Richmond
Current Results
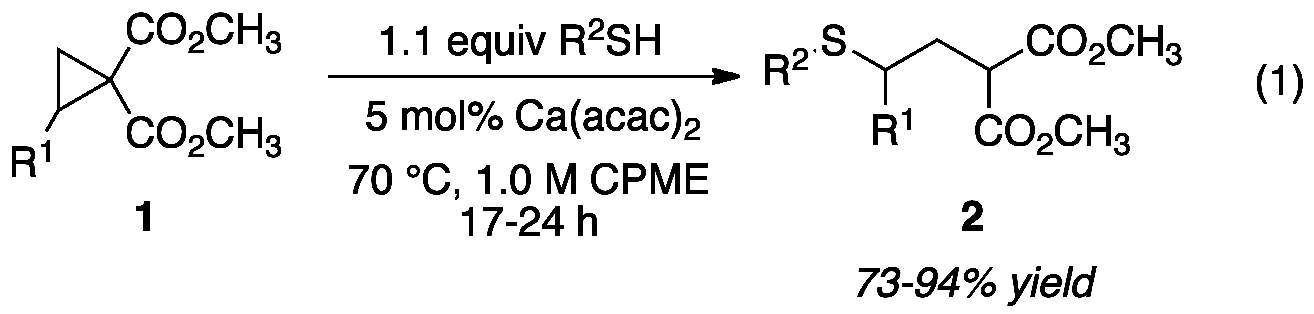
Development of methodology for the addition of unactivated thiols to donor-acceptor (DA) cyclopropanes has been completed. After a catalyst screen, we found that commerically available calcium acetylacetonate (Ca(acac)2) facilitated the addition of 4-chlorothiophenol, without prior activation or exogenous base, to cyclopropane
We have expanded upon our calcium-catalyzed addition reactions by developing catalytic C–C bond forming reactions. Two classes of carbon nucleophiles are of particular interest and are being tested as substrates in the HCA reaction: (1) electron-rich aromatic and (2) heteroaromatic compounds. We began our investigation into these catalytic Friedel-Crafts reactions by examining the addition of 1-methylindole to cyclopropane
While reaction development has begun with malonate cyclopropanes, DA cyclopropanes with two different geminal electron-withdrawing groups would be extremely useful. Such cyclopropanes have additional opportunities for derivatization. For example, the products from addition to hemi-malonate cyclopropanes can undergo decarboxylation to leave one carbonyl. Nitrocyclopropane carboxylates can be selectively reduced and hydrolyzed to produce the corresponding amino acid, which can then undergo decarboxylation to give the amine or further reduction to give the amino alcohol. These two classes of DA cyclopropanes will be examined in due course.
We are beginning to expand our methodology beyond cyclopropanes bearing two electron-withdrawing groups. These new classes of substrates include cyclopropyl ketones, imines, and esters. It is anticipated that these substrates will be less reactive than their malonate counterparts, especially in the HCA reaction where the competing 1,2-additions may prevail. For the initial investigations, (2-phenyl)cycalopropyl phenyl ketone is being used as the model substrate and has been synthesized according to literature procedures; a synthetic protocol allows for flexibility in the aryl substitution. Preliminary results for the addition of 1-methylindole show modest conversion.
Impact
ACS-PRF support has enabled four research students to have a summer research experience and a dozen more to have supplies for research during the school year. Some of these students have graduated and are continuing their studies toward PhD, MD, and DDS degress. These students have made tremendous progress on their projects and were able to gain experience in reaction optimization, synthesis, purification methods, instrumentation usage, and data analysis. Their results were communicated in publications and numerous presentations including an award-winning poster presentation at 2012 SERM-ACS meeting.
[1] Braun, C. M.; Shema, A. M.; Dulin, C. C.; Nolin, K. A., "Homologous Conjugate Addition of Thiols to Electron Deficient Cyclopropanes Catalyzed By a Calcium(II) Complex" Tetrahedron Lett. 2013, 54, 5889–5891. j.tet http://dx.doi.org/10.1016/let.2013.08.102
[2] Dulin, C. C.; Murphy, K. L.; Nolin, K. A., "Calcium-Catalyzed Friedel-Crafts Addition of 1-Methylindole to Activated Cyclopropanes" <i.Tetrahedron Lett.2014, 55, 5280–5282. http://dx.doi.org/10.1016/j.tetlet.2014.07.108