Reports: UNI352181-UNI3: Redox-Active Aluminum Complexes for Application in Carbonyl Reduction
Christopher R. Graves, PhD, Albright College
Part 1 — Synthesis and Characterization of Aluminum-a-Diimine Complexes
Diimine ligands were synthesized and characterized and their coordination chemistry to aluminum across several oxidation states was investigated. In the previous year of this grant, we demonstrated that Al-a-diimine complexes could be prepared incorporating both the singly- and doubly-reduced forms of the ligand. Diimines of the type Ar-N=C(CH3)-C(CH3)=N-Ar (Ar = 2,4-iPr2-C6H3 (LDipp) and Ar = 2,4,6-Me3-C6H2 (LMes)) were investigated. We completed this part of the study in the 2013-14 academic year and published our results in Inorganic Chemistry.
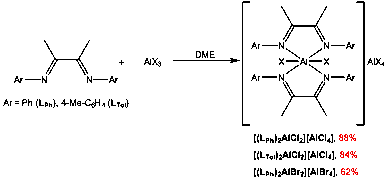
We continued the synthesis of Al-α-diimine complexes to include those supporting neutral diimine ligands. We prepared ligands without alkyl groups in the 2,6-positions in the aromatic side arms, Ar = C6H5 (LPh) and Ar = 4-Me-C6H4 (LTol). Reaction of equimolar amounts of either LPh or LTol with AlX3 (X = Cl or Br) in DME gives [(diimine)2AlX2][AlX4] in good yield (Figure 1). Products crash out of solution as pale yellow powders applicable to further reaction chemistry.
Figure 1. Synthesis of Al-a-diimine complexes of neutral diimine ligands.
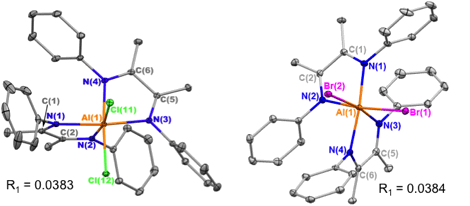
The Al-α-diimine complexes were characterized using various techniques, including multinuclear NMR spectroscopy, X-ray crystallography, Density Functional Theory, and electrochemistry. The solid-state molecular structures (Figure 2) show that the aluminum ion in the [L2AlX2]+ cation is octahedral with the two X ligands in a cis arrangement. Bonding metrics indicate that the ligands have been coordinated in the neutral form. The full geometry of the [(LPh)2AlCl2]+ cation was optimized using DFT and the theoretical bond distances and angles are in good agreement with those obtained in the X-ray analysis.
Figure 2. Solid-state structures of [(LPh)2AlCl2]+ (left) and [(LPh)2AlBr2]+ (right) cations. Ellipsoids are at 30% and H atoms are omitted for clarity.
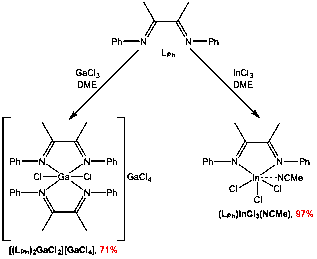
We have also investigated the coordination chemistry of the LPh ligand to Ga and In. Reaction of LPh with GaCl3 affords [(LPh)2GaCl2][GaCl4], while the analogous reaction with InCl3 gives the (LPh)InCl3(NCMe) complex (Figure 3), both after crystallization from acetonitrile/ether at -25 °C.
Figure 3. Synthesis of neutral a-diimine complexes of gallium and indium.
Part 2 — Synthesis and Characterization of Aluminum-Nitroxide Complexes
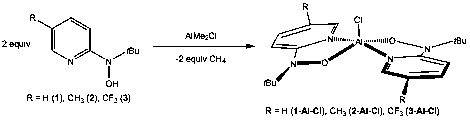
We have continued our investigation on the coordination chemistry of 2-pyridyl hydroxylamines to aluminum. We demonstrated last year the reaction of two equivalents of ligand precursor 1-3 with AlMe2Cl affords the [?2-Npy,O-py-2-N(t-Bu)O]2AlCl 1-Al-Cl-3-Al-Cl in excellent yield (Figure 4).
Figure 4. Synthesis of aluminum complexes of the <spanso-bidi-;">2-pyridyl hydroxylamine ligands.
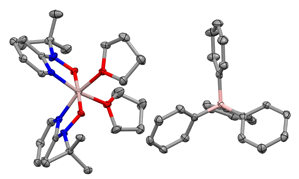
In this year, we have continued our characterization of the [pyNO]2AlCl complexes and have begun to investigate their reaction chemistry, namely in the substitution of the chloride atom. Our preliminary results demonstrate that the chloride ligand can be substituted with both an amide (NTMS2) and alkoxide (Ot-Bu) group. Additionally, reaction of 1-Al-Cl with AgBPh4 gives the complex [pyNO]2Al(THF)2][BPh4] (Figure 5).
Figure 5. Solid-state structures of [pyNO]2Al(THF)2][BPh4]. Ellipsoids are at 30% and H atoms are omitted for clarity.
Summary: In the second year of this project we have prepared and characterized two different classes of aluminum complexes implementing redox active ligands. We have begun to investigate the reaction chemistry. Our current focus is on fully characterizing the substitution products in the Al-nitroxide chemistry, including an investigation of the electrochemical behavior of the reaction products. We are also studying the reaction chemistry of the Al-α-diimine complexes with reductants.
Two undergraduate research students were supported by this PRF in the 2013-2014 year. This research opportunity has exposed the students to a diverse skill set, including the synthesis and handling of air-sensitive compounds and several characterization tools. This training will have a huge impact for future opportunities for the students, as they will be more competitive when applying for graduate school in Fall 2014.
We continued to disseminate the results of this project through presentations at regional and national meetings. Both the PI and the students working on the project have attended meetings, including posters at several local meetings, the 247th ACS National Meeting in Dallas, the 248th ACS National Meeting in San Francisco, and the 2014 Gordon Conference on Inorganic Chemistry. We also had our first paper published (Cole, B. E.; Wolbach, J. P.; Dougherty, W. G.; Piro, N. A.; Kassel, W. S.; Graves, C. R. "Synthesis and Characterization of Aluminum-a-Diimine Complexes Over Multiple Redox States" Inorg. Chem. 2014, 53, 3899-3906.)