Reports: DNI353254-DNI3: Electronically Responsive Ligands: Toward Two-Electron Reactivity with Earth-Abundant Transition Metals
Christopher C. Scarborough, PhD, Emory University
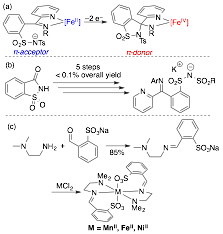
Figure 1. (a) Proposed ERL target, which we demonstrated (b, Ar = Mes), albeit in impracticably low yield. (c) Alternative ligand explored.
During the above studies, other results in our lab drew the PRF project in a new direction. In efforts to access a Co–oxo supported by sulfonamidate ligands via O-atom transfer to CoII (Figure 2a), we were able to demonstrate reversible O-atom transfer not to CoII, but to the sulfonamide oxygen to form the first peroxysulfonimidate. This peroxysulfonimidate, which is competent for selective oxidation of benzene to phenol, follows the conceptual blueprint of ERLs and provides a new concept for accessing two-electron reactivity from a Co species that is electrochemically susceptible only to one-electron oxidation. That is, the two-electron reactivity preference of the peroxysulfonimidate is a reactivity mismatch with the 1-electron-reactive CoII ion, and affords overall two-electron reactivity.
Simultaneous to the peroxysulfonimidate discovery, we developed the first synthesis of 1,4,7-tri-tert-butyl-1,4,7-triazacyclononane (t-Bu3tacn, Figure 2b).1 We targeted this ligand because of its potential to support aerobic reactivity at cuprous ions without intramolecular ligand oxidation2 to enable intermolecular aerobic oxidation catalysis. To take advantage of this CunO2 reactivity, we reasoned that the reactive center should be designed to prevent release of oxygen- and/or carbon-centered free radicals, and that generating CunO2 species within a hemilabile cage would achieve this. Building such a cage using sulfonates opens the possibility that peroxysulfonimidate-like persulfonyl species are accessible from Cu/O2, and may be selective for two-electron aerobic oxidation pathways (Figure 2c). Before we can build such novel caging ligands, we needed to establish whether the t-Bu groups in t-Bu3tacn are resistant to oxidation by a CunO2 core, work that was supported by the PRF.
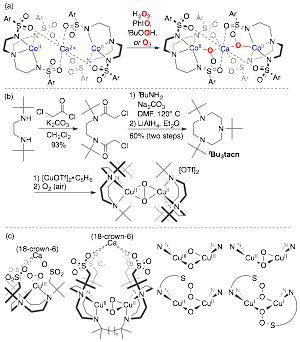
Figure 2. (a) Our demonstration of O-atom transfer to sulfonyl oxygens to form the first peroxysulfonimidate (right). (b) Our recently reported first synthesis of t-Bu3tacn, and demonstration that the t-Bu groups are stable to a Cu2O2 core. (c) New targets that form cages designed for selective substrate oxidation, with possible Cu2O2 arrangements.
In what will be our first PRF-supported publication, we have shown that the t-Bu groups of t-Bu3tacn are uniquely resistant2 to intramolecular oxidation, and have fully characterized a Cu2O2 adduct with unprecedented stability to intramolecular oxidation (Figure 2b). This species is reactive for intermolecular substrate oxidation at room temperature, which starkly contrasts related CunO2 adducts.2 [(t-Bu3tacn)CuI(OTf)] is a competent catalyst for the aerobic oxidation of benzoin to benzyl and 3,5-di-tert-butylcatechol to the corresponding quinone, and these results provide the foundation to build novel caging ligands that control substrate oxidation to achieve selective aerobic C–H oxidation.
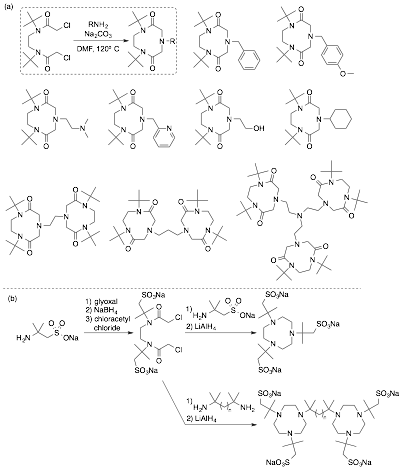
Figure 3. (a) Ligands we have accessed by our novel R3tacn route (Figure 2b). (b) Proposed synthesis of ligands required for studies shown in Figure 2c.
In order to access the caging ligands shown in Figure 2c, we needed to establish the generality of our t-Bu3tacn synthesis. Figure 3 highlights the generality of our route, as every compound shown was previously inaccessible. Of especial relevance is cyclization with ethylene diamine, 1,3-diaminopropane, and tris(2-aminoethyl)amine (tren) to afford bridging ligands, as these demonstrate the viability of the bis-tacn targets shown in Figure 2c. Our synthetic efforts are now being applied to the construction of the caging ligands shown in Figure 3b. The second year of PRF funding will focus on the synthesis of caged CuI complexes shown in Figure 2c, as well as studies of their aerobic reactivity, with emphasis on establishing whether substrate oxidation is accompanied by generation of unselectively reactive free radicals, or if reactivity is controllable using cages, analogously to enzymes and porous heterogeneous catalysts.
PRF funding has been critical in supporting the PI's developing career by funding our recent work on aerobically reactive copper complexes supported by novel triaazacyclononane derivatives. These results will serve as the foundations for the PI's research program that seeks to develop novel catalysts for selective aerobic hydrocarbon oxidation.3 Furthermore, the PRF is enabling the PI to pursue an aspect of his research that is unique in the field of oxidation catalysis, namely that peroxysulfonimidates (and perhaps related persulfates) are accessible using base metals and provide a new conceptual framework for the development of two-electron O-atom transfer reactivity. The expansion of this peroxysulfonimidate concept to aerobic copper chemistry will be enabled by PRF funds, and may be a critical advance in selective oxidation catalysis, as this may enable the selective aerobic oxidation of benzene to phenol using base-metal catalysts, which has been described both as a "holy grail"4 and one of the top-ten most difficult problems in catalysis.5 The students supported by the PRF funds gain critical training in synthetic inorganic and organic chemistry, catalysis, and contribute to one of the critical challenges in catalysis facing their generation, selective hydrocarbon oxidation.3
[1] Thangavel, A.; Wieliczko, M.; Bacsa, J.; Scarborough, C. C. Inorg. Chem. 2013, 52, 13282-13287.
[2] Lewis, E. A.; Tolman, W. B. Chem. Rev. 2004, 104, 1047-1076.
[3] Cavani, F. Catalysis Today 2010, 157, 8-15.
[4] Wittcoff, H. D.; Reuben, B. G.; Plotkin, J. S. In Industrial Organic Chemicals; 3 ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2013, pp 330-331.
[5] Alonso, D. A.; Nájera, C.; Pastor, I. M.; Yus, M. Chemistry – A European Journal 2010, 16, 5274-5284.