Reports: ND952789-ND9: Realizing the Selectivity of Exothermic Partial Oxidation Reactions
Anthony G. Dixon, Worcester Polytechnic Institute
Ethylene epoxidation is one of the most important industrial reactions, many studies have been done to understand the reaction and improve the process selectivity. It is reported that annual benefit of 1% increase in selectivity is around several tens of million dollars (Stegelmann et al., 2004). In this work, we coupled detailed microkinetics and CFD to determine the effects of flow and the fixed bed configuration on the reaction.
A microkinetics model for ethylene epoxidation has been proposed by Linic and Barteau (2003). In this kinetics model there are three species, as well as fractions of empty, oxygen, ethylene, and ethylene oxide covered surface sites. Solving the microkinetics model under steady state conditions, we concluded that oxygen dissociation and surface reaction are rate determining steps. Using the steady state approximation and J. A. Dumesic analysis based on De Donder relations (Dumesic et al., 1999), we developed an analytic solution for the microkinetics model. This model includes change of rate determining steps under different reaction conditions.
After determining general kinetics for the epoxidation and specifying surface coverages based on partial pressures and temperature, we were able to write the equations for mass, momentum, and energy using the solid particle method (Dixon et al., 2010) that was developed during a previous ACS-PRF grant. User-Defined Scalars are applied to define species mass fractions inside the catalyst particle.
In our model we used User-Defined Memory locations to store the calculated values of rate of reaction and fractions of the surface sites. All of the convergence criteria have been checked using defined-on-demand functions.
We examined our model by applying it to one sphere inside a tubular geometry. The equations are solved for turbulent case using the SST k-? turbulence model with inlet velocity of 1 m s-1 and equivalent molar fractions of oxygen and ethylene in the feed. All of the equations were successfully solved, and the model was able to show us coverage distribution on the surface and inside the catalyst particle.
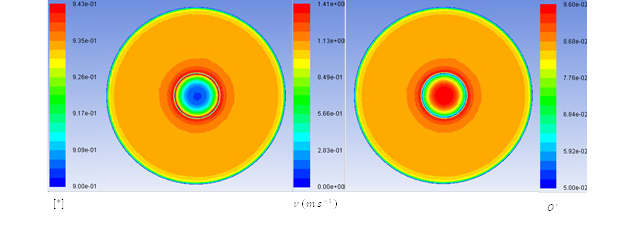
Figure 1. Contours of O* and [*] Inside the solid sphere and velocity in fluid phase inside the tube on a cross-section plane through the tube and particle.
Figure 1 shows that User-defined memory is a valid method for storing surface sites and reaction rates. Results have been compared with a full numerical solution of the model which has been proposed by Linic and Barteau (2003) and they are in good agreement.
The next step was applying our model to two spheres (rp = 3 mm) inside a narrow tube (rt = 5 mm) as the geometry for two different cases. The first case has the same conditions as above with a different inlet velocity (0.1 m s-1). An important factor is that heat of reaction of ethylene epoxidation is (relatively) low, and its effect on operating temperature is negligible for the conditions of this preliminary study, but under the industrial conditions when we have complete oxidation of ethylene and ethylene oxide as parallel reactions, heat generation would be considerable. Therefore, for the second case we ran the simulation under hypothetical conditions in which heat of reaction is multiplied by 103.
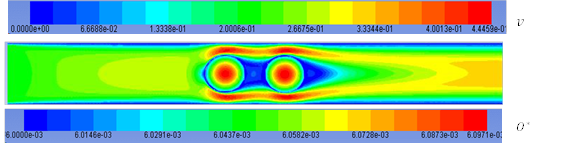
Figure 2. Contours of O* surface coverage and velocity. For Tinlet = 493 K and vinlet = 0.1 m s-1
Figure 2 depicts velocity contours in fluid phase and O* surface coverage fraction in the solid as we expected them to be. Temperature and partial pressures changes in this case are very low and because surface coverages are highly dependent on these two, they are distributed almost uniformly in this case.
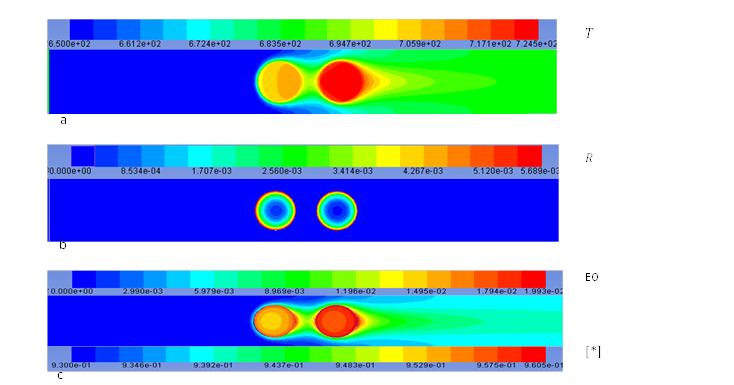
Figure 3. Contours of (a) temperature T (K), (b) reaction rate R (Kmol m-3 s-1), (c) mass fraction of EO and empty site coverages [*] on an iso-surface plane. For Tinlet = 650 K and vinlet = 0.1 m s-1 and ΔHrxn = 1000 × ΔHrxnreal .
Figure 3 represents hypothetical conditions. One of the interesting results of this case is Figure 3. Part b. It is shown here that, although rate of ethylene epoxidation is increasing with temperature and second catalyst particle has a higher temperature, rate of reaction is lower there. This is due to a lower concentration of the reactants in that area. This illustrates the complexity of transport equations that have been solved in our model.
The method which has been presented here was successfully used to track surface site species along with all other species and properties of a catalytic reaction during a CFD simulation. The impact of this research is that in applying this method to more comprehensive kinetics, including parallel reactions will allow us to have a better understanding of the effects of flow and fixed bed configuration on surface sites distribution and design of better catalysts. It is also important to mention that we plan to expand this method to study selectivity and try to improve it. Funding of this research is leading the PI’s work into new areas involving microkinetics and CFD, and their application to partial oxidation reactions. The PhD student, Behnam Partopour, has been able to develop methodology that he will be using throughout his PhD work and has developed expertise in the use of CFD that will be of great benefit in his future career.
References:
Dixon, A. G.; Taskin, M. E.; Nijemeisland, M.; Stitt, E. H., Ind. Eng. Chem. Res. 2010, 49, 9012.
Dumesic, J. A., J. Catal. 1999, 185, 496.
Linic, S.; Barteau, M. A., J. Catal. 2003, 214, 200.
Stegelmann, C.; Schiodt, N. C.; Campbell, C. T.; Stoltze, P., J. Catal. 2004, 221, 630.