Reports: UR550594-UR5: Fundamental Studies of Diffusion and Retention on Porous Polymer Monolith Stationary Phases Used in Capillary Electrochromatography
Michelle Marie Bushey, Trinity University
We investigate organic porous polymer monoliths (PPMs) used for capillary electrochromatography. These phases are different from traditional silica based reversed phase stationary phases. We study fundamental properties of PPMs so that better phases can be developed and so that these phases, and by comparison traditional phases, can be better understood. We investigate the analyte, mobile phase, and stationary phase interactions through a series of experiments that probe the thermodynamics of retention and the relationship between analyte diffusion and retention. Our primary focus has been on lauryl acrylate. Mobile phases are mixtures of acetonitrile and aqueous buffers.
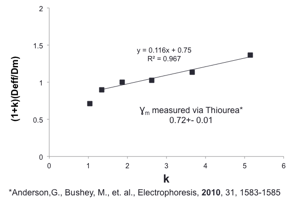
The diffusion – retention relationship has been
described by eq. (1), first proposed by Knox (
A simple rearrangement is shown as eq. (2). This indicates
that a plot of the parameters on the left versus the retention factor should
yield a straight line where the slope provides the kinetic parameter and the
intercept is the obstruction factor. All literature reports to date have shown
negative deviations. No linear results have been presented. Our data shows a
linear fit. Using the peak parking method we have used phenol as a test
compound to verify our procedures for measuring diffusion coefficients. We have
repeated our data set on three separate polymer batches. The data shown in Fig.
1 is an average all three data sets. Now that three replicate data sets have
been obtained, a manuscript is being drafted for submission.
The explanation for the linear fit is that these polymers have
a relative lack of mesopores as indicated by a very
low internal porosity. The total porosity measurement of 0.85 is consistent
with literature values for silica monoliths and the external porosity is 0.83 is
high compared to other phases. This is due to the internal porosity measurement
of 0.12, which is well below the typical reported values of 0.4-0.5 for
traditional and silica monoliths.
The thermodynamics of retention are also investigated. The relationship
between retention and temperature is described by the vant
Hoff equation, eq (3).
ln k = The enthalpy and entropy of retention can both be obtained
if the phase ratio (F) is determined. Our value of phase ratio is 0.305.
Furthermore, we have found the phase ratio to be constant across the
temperature range of interest. The studies of the thermodynamics of retention
have been completed for a temperature range of 30-65 degrees on three polymer
batches for two different mobile phases. Studies on a third mobile phase have
been completed on one polymer batch. The results the enthalpy
of retention are shown in Table 1 for the three mobile phases. Retention
increases as the enthalpy becomes more negative.
The data for the entropy of retention is shown in Table 2. It
is interesting to note that for the mobile phase of 75% acetonitrile and 25%
aqueous buffer, as well as for 65% acetonitrile and 35% aqueous buffer, the
retention is clearly enthapically driven since the
entropy becomes increasingly more negative as compounds become increasingly
hydrophobic and retention increases. This is not the case if the amount of acetonitrile
is decreased to 55%. In this case the entropy becomes more positive as
retention increases. This interesting result is being studied further by
examining the retention – temperature relationship with other mobile
phases.
Trinity University has recently acquired a scanning electron
microscope. Some images of polymers have been obtained and we are comparing the
dimensions and feature of polymer prepared in different manners. For instance
we are comparing the structural features of polymers prepared with and without electroosmotic flow carriers so that the separation
performance characteristics of these polymers can be correlated with the
structural features. An example image is provided in Fig. 2.
During the past grant year, ten individual students have
participated. ACS-PRF has supported two of these students with summer stipends
in 2014, others were supported by other funding
mechanisms or conducted research during the academic year for course credit.
Three former undergraduates are in graduate school (Chemistry or other STEM),
one is employed as a chemistry lab technician, one is employed overseas, one is
in pharmacy school, one has entered the army, one plans
to study patent law. Eight others have yet to graduate.
Students gave presentations at the 247th ACS
national meeting in 2014 and at Pittcon 2014.
Additional presentation abstracts have been submitted for spring 2015 meetings.
An invited talk was presented at the Latin American Capillary Electrophoresis
Symposium in Lima, Peru in Nov. 2013. In March of 2014, the PI received the
2014 Distinguished Scientist Award from the Texas Academy of Sciences.
Figure 1. Knox plot with three replicate polymer batches.
Figure 2. SEM of Lauryl acrylate polymer.
Gold coated
Table 1. Enthalpy of Retention
ΔH (kJ/mol) 75% ACN: 25% Tris base 65% ACN: 35% Tris base 55% ACN: 45% Tris base Toluene -3.9 (0.5) -4.3 (0.3) -5.5 Ethyl Benzene -4.5 (0.5) -4.8 (0.3) -6.0 Propyl Benzene -5.5 (0.6) -5.6 (0.3) -6.8 Butyl Benzene -6.6 (0.6) -6.6 (0.3) -7.7 Amyl Benzene -7.7 (0.6) -7.5 (0.4) -8.6 Table 2. Entropy of Retention
ΔS (J/mol K) 75% ACN: 25% Tris base 65% ACN: 35% Tris base 55% ACN: 45% Tris base Toluene -3.6 1.0 6.1 Ethyl Benzene -3.4 2.1 7.8 Propyl Benzene -4.1 2.5 8.8 Butyl Benzene -4.6 2.6 9.7 Amyl Benzene -5.9 2.7 10.5