Reports: UR1050635-UR10: Synthesis of Crystalline Porous Materials with Functional Open Metal Sites
Xianhui Bu, PhD, California State University (Long Beach)
In this funding period, the PI’s group developed a new strategy for synthesizing cluster-based lithium metal-organic frameworks (Li-MOFs) by using carboxylates as crosslinking ligands. Prior to this work, there were a number of reported Li-carboxylate MOFs. However, the generally observed structural pattern in these known MOFs is that Li+ tends to form polymeric Li-O chains or layers with carboxyl groups. So far, no discrete Li clusters have been observed in the Li-carboxylate system. Because MOFs constructed from discrete inorganic nodes are often more porous than those containing polymeric inorganic building blocks, for the simple reason that organic spacers are usually larger than inorganic spacers, the development of new lithium clusters in Li-carboxylate system is of considerable interest.
For Li+, the clustering could provide a new route for synthesizing Li-MOFs from more negative carboxylate ligands. In this work, the PI’s group created an interesting system by using highly negative organic anions, BTC3- and BTB3- (H3BTC=1,3,5-benzenetricarboxylic acid, H3BTB = 1,3,5-tri(4-carboxyphenyl)benzene), to promote the clustering of lithium ions. Indeed, such a method is effective and the formation of a highly unusual lithium cluster, square-planar Li4 tetramer, as well as a Li2 dimer was observed. It is worth noting that despite their simple compositions, no Li-BTC or Li-BTB MOFs were known prior to this work. These two Li-MOFs exhibit highly open architecture and Li-BTB exhibits the highest CO2 uptake capacity among known Li-MOFs.
One of the new phases synthesized (denoted as CPM-45, CPM = Crystalline Porous Material) crystallizes in the non-centrosymmetric cubic symmetry. Its most novel feature is the square-planar Li4 cluster. It is worth noting that square-planar clusters are also known for divalent metal ions such as Co2+, however, these known square-planar clusters contain an oxo (O2-) or hydroxyl (OH-) species at the center that helps to assemble metal ions together. The Li4 tetramer reported here is different and consists of only four lithium ions. From the viewpoint of self-assembly, such metal-only tetramer is expected to be more difficult to form.
Each edge of the square (i.e., two Li sites at the corners) is bridged by a –COO group, which gives a neutral Li4(COO)4 unit. This Li4(COO)4 unit in CPM-45 has two remaining coordination sites per Li for a total of 8 coordination sites. Very elegantly, it only takes two additional –COO groups, located above and below the Li4 square, to complete all these 8 coordination sites, resulting in the dinegative [Li4(COO)6]2- building blocks. In [Li4(COO)6]2-, four edge-connected BTC ligands alternate above and below the Li4 plane, while two face-connected BTC ligands are orthogonally oriented to each other. The resulting (3,6)-connected net in CPM-45 forms open channels along the cubic [111] direction. The free volume accessible to guest species is as high as 50.4%, most of which, however, are occupied by NH2(CH3)2+ serving as extra-framework charge balancing species. The remaining void space (about 12%) is occupied by disordered solvent molecules.
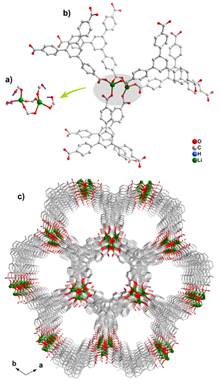
The unprecedented Li4 tetramer in the Li-BTC system highlights the potentially rich cluster chemistry of Li-MOFs, which is further proven with the discovery of the unprecedented Li2 in the Li-BTB system. Similar to Li4 tetramer in CPM-45, each Li2 dimer in CPM-46 also acts as 6-connected node linked by tritopic BTB ligands to form a new (3,6)-connected 3D framework with 1D open channels along the c-axis. Four out of six carboxylic groups around each Li2 dimer are protonated (i.e., COOH) and they bond to Li+ ions in the monodentate mode, while the remaining two carboxylic groups are deprotonated (i.e., COO-) and they bond to Li+ ions in the bidentate mode, leading to the formation of the dimeric Li2 SBU in CPM-46 (Figure 1). Up to now, though various dimeric metal clusters have been employed to create a large number of MOFs, dimeric Li2-based MOF remains unknown prior to this work. CPM-46 also exhibits a porous framework with 46% void space.
Figure 1. a) The dimeric Li2. b) Coordination mode between Li2 and its adjacent six BTB ligands. c) View of 3D framework along [001] direction, showing 1D channels.
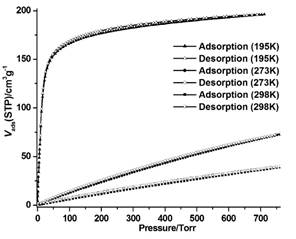
Since CPM-46 has a neutral framework with no extra-framework cations, gas sorption experiments were performed with CPM-46. As shown in Figure 2, the sorption isotherms obtained with CO2 at 195 K revealed a typical type I adsorption curve. The CO2 sorption increases abruptly at very low pressures and reaches a maximum mass uptake of 196 cm3/g at 1 atm. The sorption isotherm data were fitted to the BET equation to give a BET surface area of 592 m2/g. Calculations by fitting the adsorption data to the Dubinin–Radushkevich equation gave a microporous surface area and a pore volume of 1784.2 m2/g and 0.359 cm3/g, respectively. Additionally, at atmosphere pressure, the CO2 uptakes of CPM-46 at 273 K and 298 K reach 72.5 cm3/g and 39.3 cm3/g, respectively, which is, to our knowledge, the highest among known Li-MOFs.
Figure 2. Adsorption and desorption isotherms of carbon dioxide at various temperature by CPM-46.
The research supported by this PRF grant made a significant impact on the PI's project development and on the careers of students involved. One graduate student, Matthew Shimazu, is working on his MS thesis (only MS degree is offered on campus) through participating in this PRF-supported project. His MS thesis project on novel Li-MOFs continues the success that the PI’s group achieved in this funding cycle. In fact, the success in this funding cycle paved the way for his MS thesis work. He is making an excellent progress towards his MS thesis. An undergraduate student, Xinh Thuong Trieu, was supported by this PRF grant during her senior year, which helped her successfully obtained the BS degree in chemistry. Another undergraduate student, Shawn Kirby, completed his Honors Program Thesis Project in the University Honors Program and he has now entered the PhD program at UC Irvine. It is expected that this PRF program will continue to help promote the careers of undergraduate and graduate students by supporting their research at the PI’s group.