Reports: UR451031-UR4: The Kinetics of Aggregating Dyes
Peter J. Collings, PhD, Swarthmore College
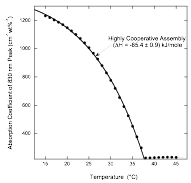
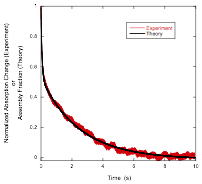
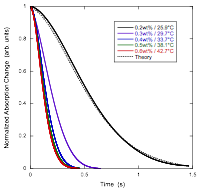
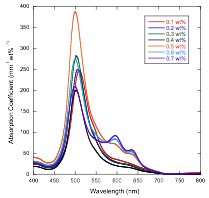
The Second Step of Assembly in IR-806
The Assembly Processes in Pinacyanol
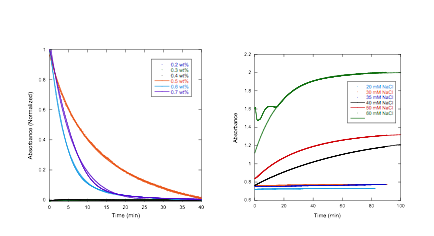
Acetate Whereas solutions freshly prepared from powder with
concentrations at or above 0.3 wt% showed absorption
changes upon dilution (indicating that large assemblies were present), after
waiting a day, only solutions with concentrations at or above 0.5 wt% showed absorption changes upon dilution. [1] Smulders, M. M. J.; Nieuwenhuizen, M. M. L.; de Greed, T. F. A.; van der Schoot, P.; Schenning, A. P. H.
J.; Meijer, E. W. [2] v. Berlepsch,
H.; Ludwig, K.; Bottcher, C.;