Reports: UR951984-UR9: Fluid Flow and Solute Migration in Supercritical Fluid Chromatography
Donald P. Poe, PhD, University of Minnesota (Duluth)
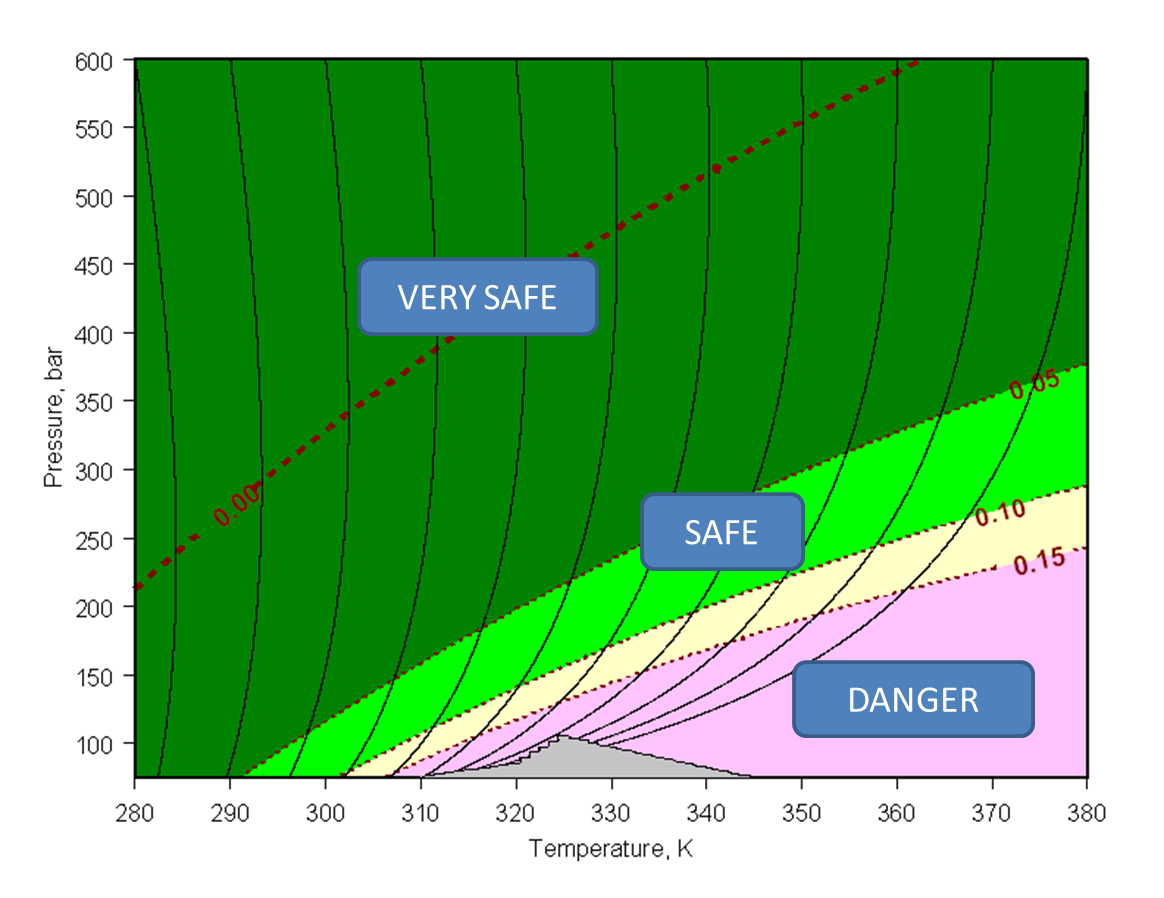
Figure 1. Operating zones in SFC using 95% CO2 / 5% methanol as mobile phase. Column: 250 x 4.6 mm ID with 5-micron Phenomenex Luna-C18 particles; solute: octadecylbenzene; thermal environment: forced air. Dashed red lines show constant Joule-Thomson coefficient value in K/bar.