Reports: UNI753340-UNI7: Tailoring the Chemical Structure of Conducting Polymers and Dopants To Enhance Electromechanical Actuation
Amanda R. Murphy, PhD, Western Washington University
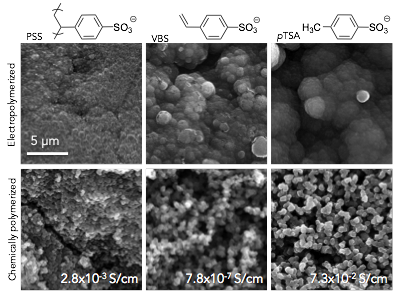
The SEM images of the films and pellets produced are shown in Figure 1. Consistent with literature reports, electropolymerized PSS-doped films exhibited a smoother surface, while the pTSA films were much rougher and had the largest nodules. Elemental analysis using EDX showed that the mean sulfur content was similar for all samples (6-7wt%). Chemically polymerized samples all contained aggregates of distinct, rounded particles. Mean particle size was similar for the PSS and pTSA samples (0.40 ± 0.11 vs. 0.43 ± 0.09 μm), while the VBS particles were smaller (0.32 ± 0.06 μm), and more jagged. Contrary to the data collected for electropolymerized films, the PSS-doped pellet exhibited much higher sulfur content than VBS or pTSA-doped pellets (11.2% vs. 5.2% and 5.1% respectively), likely due to the difficulty in rinsing away the excess due to the large size of the polymer. The film and pellet morphologies of the VBS-doped samples are intermediate between the PSS and pTSA samples, suggesting good incorporation of the VBS dopant into the PPy.
Figure 1. SEM images of electropolymerized films and pellets (scale is the same for all images).
However, the conductivity of the VBS samples is surprisingly low (Figure 1). We are currently looking into the cause, and reaction conditions are being further optimized to increase the conductivity. We are also evaluating other polymerizable dopants including vinyl and allyl sulfonic acid, and 2-acrylamido-2-methyl-1-propanesulfonic acid. These molecules were chosen to compare how small aliphatic vs. aromatic vs. bulky aliphatic sulfonic acid groups will effect the physical and mechanical properties of the final composite materials.
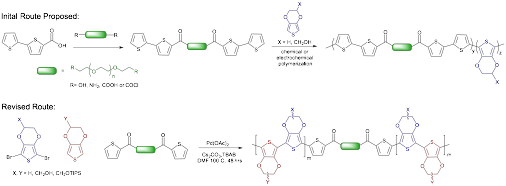
Synthesis of a series of copolymers containing PEDOT-OH and PEO segments was initially proposed as shown in Scheme 1. We were able to synthesize bithiophene-terminated macromonomers linked by ethylene glycol segments containing 4, ~67 and ~220 repeat units using DIC-activated esterification reactions. However, copolymerization of this macromonomer with either EDOT or EDOT-OH proved troublesome using both chemical and electrochemical methods. Attempted co-polymerization reactions with the shorter PEO derivatives resulted in insoluble powders that had poor incorporation of the PEO-containing macromonomer, while the larger PEO derivatives produced only short oligomeric species with poor conductivity.
Scheme 1. Initial and revised copolymer synthesis route.
Since it has proved difficult to produce the desired copolymers using our initial synthesis method, we have redesigned our scheme to utilize direct arylation polymerization reactions that have recently appeared in the literature. Here the only requirement is that one monomer be dibrominated, but the reaction is otherwise tolerant of a variety of functionalized monomers, potentially allowing for facile incorporation of the PEO-containing macromonomers. Protection of the EDOT-OH monomers with triisopropylsilane (TIPS) has been found to increase reaction compatibility, and provide additional solubility, which was an issue in our initial scheme. Initial polymerization results have been very promising, so this route will be further pursued this year.
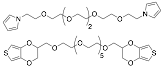
In addition to the synthesis of PEDOT-OH / PEO copolymers, we have been evaluating different methods to crosslink the reactive –OH side chains of PEDOT-OH in order to manipulate the mechanical properties of the polymer. We have successfully synthesized pyrrole and thiophene-based crosslinkers (Figure 2) that contain monomers connected by a hexaethylene glycol spacer. As shown in Table 1, addition of 10% of the pyrrole crosslinker to a pyrrole electropolymerization reaction dramatically increases the mechanical properties of the resulting films, and only slightly increases the resistivity. Current work is underway to repeat this characterization with films synthesized with the thiophene-based crosslinker, and evaluate the effects on actuation performance of both.
Figure 2. Pyrrole and EDOT-OH crosslinkers.
Table 1. Properties of polypyrrole with and without 10% crosslinker added
Film |
% Strain at break |
Ultimate tensile strength (MPa) |
Young's Modulus (MPa) |
Resistivity (Ohms/sq) |
Plain polypyrrole |
3.2 |
3.8 |
117 |
9.3 ± 1 |
10% crosslinker |
9.2 |
10.5 |
265 |
17.1 ± 2 |
Since July 2013, this project has provided research opportunities for 4 undergraduates and one masters student. Six posters in total have been presented by these students on this work; three at WWU, and three at regional and national meetings, most notably the Materials Research Society meeting in San Francisco in April, 2014.