Reports: DNI653105-DNI6: Experimental Investigations of Radical-Particle Reactions Relevant to Hydrocarbon Pyrolysis
Fabien Goulay, West Virginia University
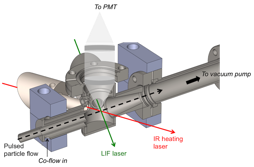
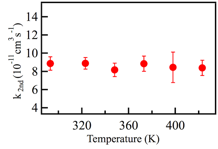
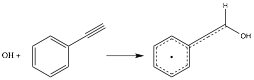
Copyright © American Chemical Society
Fabien Goulay, West Virginia University


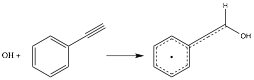
Copyright © American Chemical Society