Reports: UR752090-UR7: Novel Polymer Coupling via Thiazolidine Chemistry
Philip J. Costanzo, PhD, California Polytechnic State University
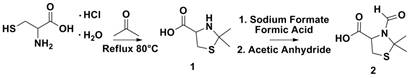
Thiazolidine chemistry is a commonly used reaction used in biological systems because the reaction requires the presence of both cysteine (a common amino acid) and an aldehyde or ketone. The cysteine residue contains multiple functionalities that be employed to prepare unique materials. We have developed a synthetic route to access three different functional points of the cysteine residue. Futhermore, protected cysteine molecules have been successfully ligated onto polymerizable monomers and have been shown to be easily deprotected in the presence of an acid source.
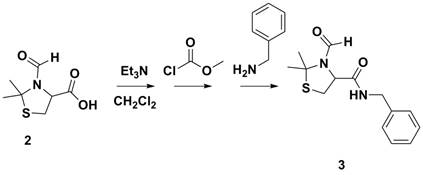
The synthesis of Next, the carboxylic acid functional group was coupling with
various amines. Scheme 2 depicts coupling with benzyl amine which was conducted
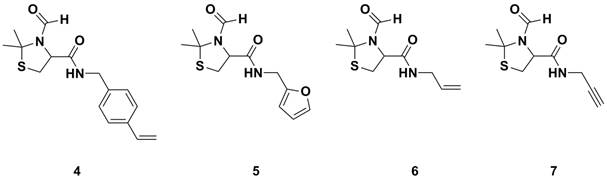
as a control experiment. Other amines, such as allyl amine and propargyl amine
have employed to allow for thiol-ene/yne chemistry, furfuryl amine to allow for
Diels-Alder chemistry and 4-vinyl benzyl amine for polymerizable monomers,
Figure 1.
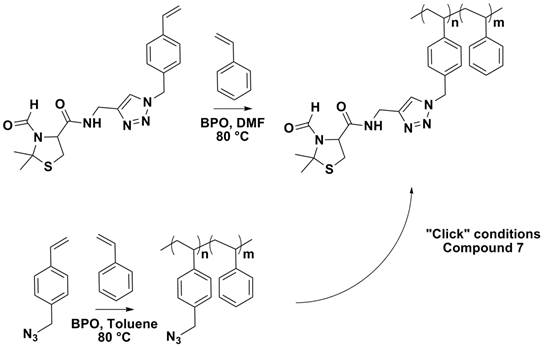
Protected cysteine residues were incorporated into polymeric
resins by the direct polymerization of compound Future work involves the modification of other resin
systems. Additionally compounds In total, four undergraduate students have completed
research from support of this award and resulted in two oral presentations
given by undergraduate students at the National American Chemical Society
meetings.