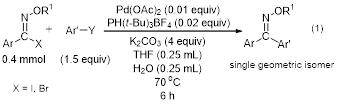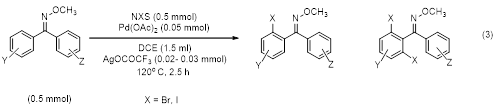Reports: UR150612-UR1: New Synthetic Methodology to Prepare Imine Derivatives in Single, Predictable Geometric Configurations
Debra D. Dolliver, Southeastern Louisiana University
Progress toward the major goals outlined in this proposal for the term of the grant are given below.
Progress toward Goal A: Establish a robust synthetic protocol to reliably synthesize single E or Z isomers of oxime ethers using a variety of imidoyl iodides and trifluoroborate salts
The PI and the undergraduate students working under her direction optimized palladium catalyzed Suzuki coupling and the palladium-catalyzed Sonogashira coupling of N-alkoxyimidoyl iodides and bromides, achieving excellent yields for a variety of coupling partners and demonstrated that this reaction proceeds with complete retention of geometry about the C=N bond (equations 1 and 2).
In addition, the palladium-catalyzed Negishi reaction was investigated. It persisted in giving an aromatic nitrile byproduct, thought to result from zinc-assisted ionization of the halide on the imidoyl halide, and the yield of the coupling product from this reaction was only moderate. All the above work resulted in a publication in the Journal of Organic Chemistry1 with nine undergraduate coauthors. Eight of these nine coauthors have proceeded to graduate school in chemistry.
As the Suzuki coupling outlined in equation 1 resulted in single geometric isomers of diaryl oxime ethers, this provided the opportunity to explore the palladium-catalyzed C-H activation/functionalization at the ortho position (equation 3). The exploration of ortho-bromination and iodination of these compounds has been completed. This work revealed that these compounds only undergo ortho halogenation on the ring trans to the N-OCH3 group and the geometry of the C=N bond is maintained during the reaction. This reaction tolerated a variety of substituents and gave good to excellent yields of the mono-ortho-halogenation product with small amounts of the di-ortho-halogenated product. Independent synthesis of a diaryl oxime ether palladacycle was also successfully performed. This palladacyle was found to produce the same mono-ortho-halogenation product when placed in the same conditions used for the catalytic reaction discussed above. This finding helps elucidate a potential intermediate for this reaction pathway. This work has just been published in Tetrahedron Letters2 with three undergraduate coauthors (all of which are now currently in graduate school in chemistry).
Additional work on the ortho alkoxylation of these diaryl oxime ethers is currently underway.
Progress toward Goal B: Investigate the above coupling technique with N-alkoxyimidoyl pseudohalides
The Suzuki coupling reactions were attempted with the N-alkoxyimidoyl tosylates [ArC(OTs)=NOR]. This reaction yielded no coupling products. Attempts at making the N-alkoxyimidoyl triflates have demonstrated that these compounds are not stable to moisture and revert back to the starting material during the work up. Because of the unreactivity of the tosylates in these reactions and the instability of the imidoyl triflates, further work in this area has been put on hold to pursue other successful lines of research.
Progress toward Goal C: Investigate the coupling technique with other N-substituted imines
Work has been initiated using hydrazonyl bromides [R1C(Br)=N-NR2R3] in palladium-catalyzed coupling reactions. To date this work has been complicated by competing cyclization reactions depending on R2 and R3. If these reactions yield some of the palladium-catalyzed coupling products, optimization of the reaction conditions will be carried out.
Progress
toward Goal D: Utilize
the established coupling reaction to make biologically-active compounds
Progress
toward the general goal of providing solid research training to undergraduates: The funds
from the PRF grant have allowed the PI to productively engage undergraduate
students in publishable research. Three of the students financially supported
by the PRF grant were co-authors on peer-reviewed publications.1,2
All three of these students are now in graduate school in chemistry (University
of Michigan, Purdue University and Louisiana State University). The PRF
support has allowed the PI to build a successful collaboration with two organic
chemists at the University of Alabama (U.A). It has allowed students to work
with both the PI and the investigators at U.A. giving them an outstanding
undergraduate research experience. Additionally, this collaboration has
allowed the PI to do more in-depth studies which have resulted in publications
in top-tier chemistry journals. References:
3.