Reports: DNI753195-DNI7: Synthesis of Multivalent and Multimodal Nanoparticles by 'Clicking-To' Multiblock Dendrimers
Jonathan G. Rudick, PhD, State University of New York at Stony Brook
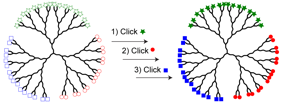
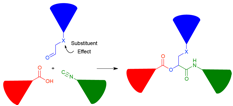
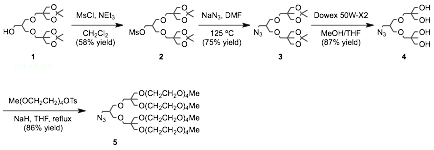
Jonathan G. Rudick, PhD, State University of New York at Stony Brook



Copyright © American Chemical Society