Reports: ND350971-ND3: Electrocatalytic Water Oxidation by Manganese Pyridinophane Complexes
Jeremy M. Smith, Indiana University
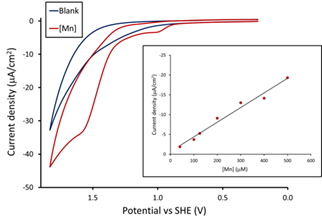
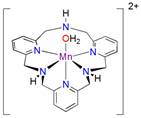
We previously reported that the cyclic voltammogram (CV) of (Py2NtBu2)Mn2+ (Fig. 1, R = tBu) in reveals a catalytic wave for water oxidation in basic solution (Fig. 1). Dioxygen formation was directly characterized by a number of methods, including cyclic voltammetry, gas chromatography and an O2 electrode, as well as indirectly through the pH change of unbuffered solutions. In addition, multiple experiments suggest that catalysis is homogeneous. Finally, the catalytic current varies linearly with the concentration of the Mn complex (Fig. 2, inset), consistent with a mononuclear catalyst for water oxidation.
A paper reporting these results was initially submitted in
July 2013. In the course of multiple rounds of revision, we conducted additional
experiments that resulted in a better estimation of the catalyst longevity. No
catalysis is observed in buffered solutions, possibly because the ions required
for these high pH buffers (e.g. phosphate) can chelate the We have initiated a collaboration with my colleague Mu-Hyun
Baik to determine the catalytic mechanism through a combination of experimental
and computational methods. Surprisingly, the computed thermodynamics for
electron and proton transfer involving water-derived ligands on the Mn complex
are not consistent with our experimentally determined data. This discrepancy may
have its origin in the irreversibility of all waves in the cyclic voltammogram
of the complex. Due to this irreversibility, the measured We previously reported that (Py3NH3)Mn2+
(Fig. 2a) shows catalytic activity for electrochemical water reduction in mildly
acidic aqueous conditions. Although the overpotential is very large ( Catalysis is observed over a pH range of 4-6.5, with
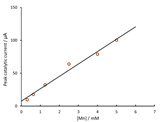
evidence for saturation in the proton concentration. The catalytic current is
proportional to the concentration of the complex, consistent with a mononuclear
catalyst (Fig. 2b). Controlled potential electrolysis in an unbuffered solution
causes the pH to increase and leads to the formation of hydrogen, as confirmed
by gas chromatography. Evaluating the catalyst performance is complicated by
apparent adsorption of the complex on the electrode, thus making comparison
with the catalyst-free water reduction difficult.
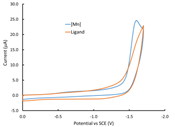
Although cyclic voltammetric measurements of the free ligand
Py3NH3 reveal that it is also a proton reduction
catalyst, it is apparent that the presence of Mn has a measurable, albeit small
influence on the catalytic performance. Thus, while the two species have the
same overpotential for electrocatalysis, the Mn complex has a greater peak
current than does the free ligand under the same conditions (Fig. 2c). We
speculate that the ligand is the critical element for catalysis and that the Mn
may preorganize the conformation to facilitate the key bond breaking and
forming events. We plan test this hypothesis by measuring catalysis in the
presence of other metal ions.
(a) (b) (c) As mentioned above, the PI has developed new collaborations
that will enhance his contribution to electrocatalysis. After a year of review
and revision, our initial paper on water oxidation was recently published in A graduate student will continue to be employed on the
project for part of the upcoming year. This research assistant support will
allow more time to be devoted to the project.