Reports: ND353349-ND3: Well-Defined Zinc and Aluminum Catalysts for Reduction of Petroleum Derived Products
Georgii I. Nikonov, PhD, Brock University
Scheme 1.
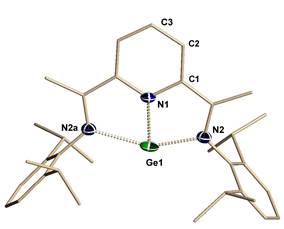
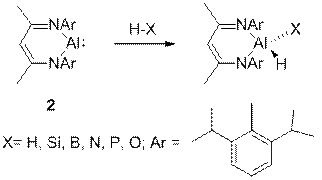
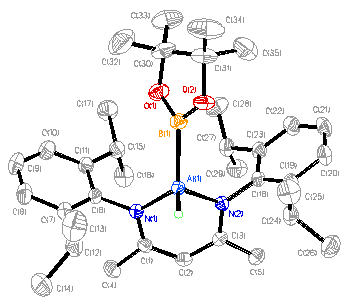
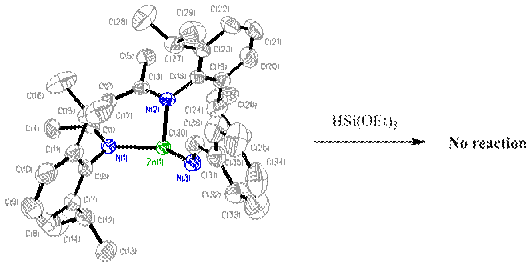
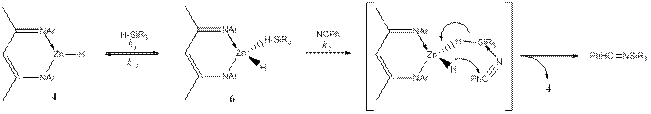
![]()
Georgii I. Nikonov, PhD, Brock University
Scheme 1.





![]()
Copyright © American Chemical Society