Reports: UR1053614-UR10: Supercritically Functionalized Nonstructures for Methane Reforming
Alevtina Smirnova, PhD, South Dakota School of Mines and Technology
The performance of the SOFC anode, where the internal reforming of methane takes place, depends on the microstructure [[1], [2]], processing conditions, and chemical composition. The microstructure of the SOFC anode defines the catalytic activity, chemical stability, and SOFC durability especially in hydrocarbon fuels, e.g. methane [[3], [4]]. Different synthesis methods (e.g. solid state reaction, sol-gel, hydrothermal treatment, and co-precipitation) have been used for the ceramic powder processing. In comparison to these methods, the combustion approach has advantages (e.g. it produces powders with high surface areas).
The major tasks of Year I were focused on establishing a foundation in SOFC anode materials processing using the glycine-nitrate combustion approach (Task 1) followed by functionalization in supercritical CO2 environment (Task 2). Additionally, the manufacturing of the test rig for SOFC performance evaluation has been completed (Task 3).
Task 1: SOFC Anode Catalyst Synthesis and Materials Characterization
In the past, many ternary and binary nickel-based anode materials such as NiO-Ce1-x Zrx O2 [[5]], Ni1-xCuxSDC [[6]], Ni + Fe + SDC [[7]], and FexCo0.5xNi0.5–SDC [[8]] were synthesized by sol-gel combustion method.
A nitrate-glycine combustion approach has been used for synthesis of three basic SOFC anode materials. In this approach, glycine performs as a fuel and is oxidized by nitrate ions. The combustion at 300-400°C is followed by an exothermic reaction between the oxidizer and the fuel resulting in temperatures 31000°C. This reaction provides the heat necessary for formation of nanocrystalline powders.
Task 1 was focused on synthesis of Nickel Oxide (NixOy), Gadolinia doped Ceria (GDC), and a mixture of NixOy and GDC in one pot synthesis. Further functionalization of these materials with metal catalyst nanoparticles using supercritical CO2 fluids is targeted in Year II.
Three solutions containing glycine as a complexing agent were prepared, specifically (a) Gadolinium nitrate and cerium nitrate (1:9 molar ratio), (b) Nickel nitrate, and (3) Nickel nitrate with gadolinium nitrate, and cerium nitrate. The solutions were evaporated at ~50°C and then heated to ~250°C to facilitate combustion. After combustion, the reaction products were collected and tested for crystal structure and total surface area.
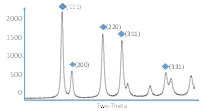
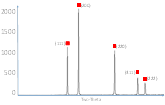
The XRD results indicate, that NiO, and Gd0.1Ce0.9O2 and a mixture of NiO (50wt.%) and Gd0.1Ce0.9O2 (50wt.%) can be successfully synthesized using combustion synthesis. For Gd0.1Ce0.9O2 the crystal structure parameters correspond exactly to those from XRD database even without additional sintering. The surface area of the compounds was evaluated from a single point BET method. The obtained BET values for the samples sintered at 1200°C were the highest for Gd0.1Ce0.9O2 (41m2/g) and lower for NiO (5.6m2/g ) and a mixture of NiO (50wt.%) and Gd0.1Ce0.9O2 (50wt.%) (6.9m2/g). The produced powders were further sintered at 900°C and 1200°C to evaluate possible changes of the crystal structure. The XRD spectra for three synthesized samples sintered at 1200°C are presented in Fig. 1. In Year 2 these materials will be functionalized using metallorganic precursors in supercritical CO2.
 |
 |
 |
| A | B | C |
| Fig. 1: XRD spectra for a) Gd0.1Ce0.9O2; b) NiO; and c) NiO (50wt%) and Gd0.1Ce0.9O2 (50wt%), each sintered at 1200°C | ||
Task 2: Supercritical CO2 Functionalization of the Anode Material
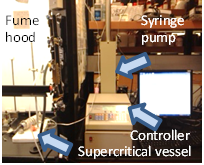
Supercritical CO2 deposition of Pt organometallic precursor within the commercial electrolyte powder Scandia Stabilized Zirconia has been performed. Two pouches of filter paper were filled with Scandia Stabilized Zirconia and Pt organometallic precursor. The pouches were then wrapped in a stainless steel mesh and placed inside the supercritical vessel (Fig. 2). The vessel was brought to 2000psi at 50°C (the conditions producing a supercritical CO2 fluid) by ISCO Teledyne syringe pump (Fig. 2). The Scandia Stabilized Zirconia (ScSZ) was analyzed using SEM and EDX.
Fig. 2: Supercritical vessel with a heating tape and sapphire view window in a fume hood (left) attached to a syringe pump (right).
Task 3: SOFC Test Rig Assembly and SOFC Testing
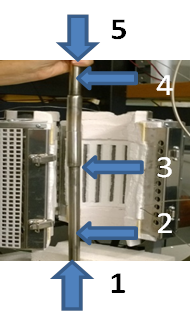
The SOFC test rig and the system for SOFC testing have been assembled. The current collectors on both sides of the coin cell were heated in a cylindrical furnace (Fig. 3) up to 600-800°C. The generated current is measured using a fuel cell 850e Teledyne test station. The button fuel cell (in-between stainless steel current collectors) is held by the weight of the top anode current collector. For better conductivity and gas distribution Ni for anode and Ag for cathode metal mesh is be attached to the stainless steel current collectors. The air/oxygen gas is be supplied from the bottom (Fig. 3-1) and the methane or hydrogen are fed from the top (Fig. 3-5).
Fig. 3: Vertical split high temperature Carbolite furnace with current collectors for cathode (2) and anode (4) providing gas supply to the SOFC electrodes and a SOFC in-between (3)
Conclusion
Year I of the project resulted in optimization of the synthesis conditions for manufacturing of anode materials using sol-gel combustion synthesis and supercritical CO2 fluid functionalization. The current collectors were designed and manufactured. Furthermore, a complete system for SOFC testing including a Scribner test station has been assembled.
In Year II the tasks will include a study of the functionalized anode materials using supercritical CO2 fluid.
Two undergraduate students Patrick Holland and Patrick Dawn from the Department of Chemistry and Applied Biological Science at SDSMT were involved in this project.
References:
[1] S. Liu , M. Koyama , S. Toh, S. Matsumura, Solid State Ionics 262 (2014) 460–464.
[2] M. V. Sandoval, A. Matta, T. Matencio, R. Domingues, G. Ludwig, M. Korb, C. Malfatti, P. Maradei, G. Gauthier, Solid State Ionics, 261(2014) 36-44.
[3] J. Xiao, Y. Xie, J. Liu, M. Liu, J. Power Sources, 268 (2014) 508-516.
[4] B.B. Patil, S. Basu, Energy Procedia, 54 ( 2014 ) 669 – 679.
[5] D. Prasad, H. Jung, H. Jung, B. Kim, K. Lee, J. Lee, Mater. Lett., 62 (2008) 587-590.
[6] Z. Xia, C. Xia, M. Zhang, W. Zhu, H. Wang, J. Power Sources, 161(2006) 1056-1061.
[7] X. Lu, J. Zhu, J. Power Sources, 165 (2007) 678-684.
[8] Z. Xie , W. Zhu, B. Zhu, C. Xia, Electrochim. Acta, 51 (2006) 3052-3057.