Reports: DNI152871-DNI1: Cyclopentannulation of Conjugated Enones Using a Vinyldiazomethane-based Reagent
Carlos A. Guerrero, PhD, University of California, San Diego
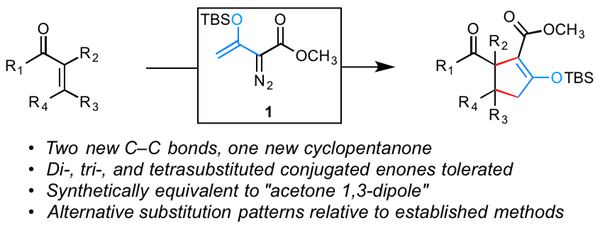
Figure 1. Concept for two-step cyclopentannulation.
Our research group's overarching goal is the generation of new chemical knowledge and the invention and development of new chemical methods using natural products as vehicles for discovery. One of our early research projects focused on the fawcettimine-class of
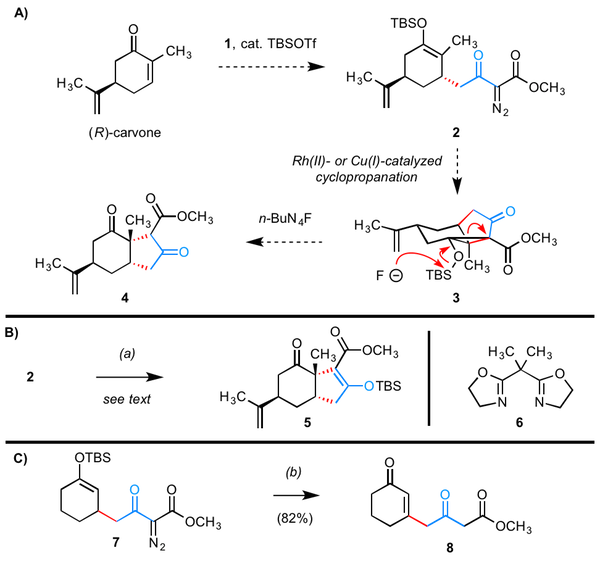
Scheme 1. Conditions and notes: (a) (CuOTf)2¥PhH (10 mol %), ligand 6 (22 mol %), toluene 55 °C, 2 h; (b) [Rh(OAc)2]2 (2 mol %), CH2Cl2, rt, 1.5 h.
We identified TBSOTf as an excellent catalyst for the first step, especially because this reagent is widely available, easily handled, and permitted fairly broad substrate scope. For the second ste, studies on the adduct of cyclohexenone and reagent 1 in cyclopropanation reactions allowed us to generalize that (1) dimeric Rh(II)-based catalysts were unsuitable for the proposed intramolecular cyclopropanation, leading instead to products of intramolecular C–H carbene insertion–fragmentation; and (2) Cu(I)-based catalysts bearing an electron rich, achiral bis(oxazoline) ligand were well suited for the desired process. Table 1 is illustrative of the substrate scope for our methodology. For the first step, the yields are generally good to excellent. Moreover, this event is responsive to pre-existing chirality in the electrophilic enone, generating products in diastereomeric ratios ranging from 5:1 to >19:1.
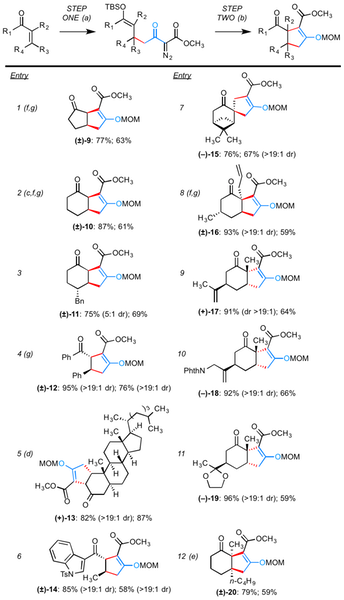
Table 1. Conditions and notes: (a) 1 (1.2 equiv), TBSOTf (20 mol %), 0 °C; (b) (i) (CuOTf)2¥PhH (12.5 mol %), ligand 6 (27.5 mol %), toluene, 55 °C;
During preliminary studies of the Cu-catalyzed annulation (second step), we observed that the crude reaction mixtures contained β-siloxy-α,β-unsaturated esters, yet these proved unstable to common chromatographic methods, including the use of Florisil. To facilitate characterization, the annulations were worked-up with n-Bu4NF and the resulting β-keto esters were alkylated (MOMCl). We highlight that these measures were taken solely to facilitate characterization, and that the annulation certainly does not require such processing. Moreover, the yields for the second step of our method include these deprotection/protection events, indicating that the yields given are minimums.
The second step of the annulation proceeds unremarkably. Generally, substrates that are competent Michael acceptors in the first step are well suited for the Cu(I)-catalyzed cyclization. One exception noted is that substrates bearing an aryl group either at either the <spanfont-family:Symbol;">a- or <spanfont-family:Symbol;">b- carbon of the conjugated enone are not amenable to the second step of the annulation, ostensibly because the intermediate Cu(I)-carbenoid reacts with the arene through Buchner-type cyclopropanation and/or related processes. In all cases examined, the use of starting cyclic enones gave
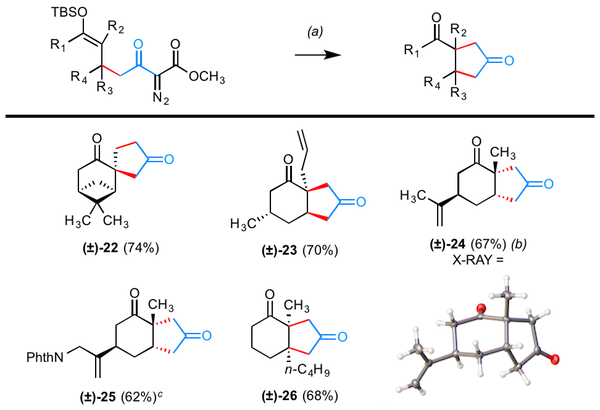
Our methodology may be diverted to give products formally equivalent to the addition of "acetone 1,3-dipole" to conjugated enones. As shown in Table 2, the crude reaction mixtures resulting from annulation can be dealkylated and decarboxylated by heating in wet DMSO or DMF. Once again, the given yields reflect material that has been subjected sequentially to the Cu-catalyzed annulation and the dealkylation–decarboxylation.
Table 2. Conditions and notes: (a) (CuOTf)2¥PhH (12.5 mol %), ligand 6 (27.5 mol %), toluene (0.05 M), 55 °C; aqueous workup; wet DMSO, 100–125 °C, 1–2 h (specific values given in Supporting Information); (b) (CuOTf)2¥PhH (15 mol %), ligand 6 (33 mol %) used; (c) DMF, 140 °C, 1.5 h, for decarboxylation.
In conclusion, we designed and developed a cyclopentannulation protocol dependent on the trifunctional reactivity of reagent 1. The two-step method operates on conjugated enones, which are common, readily obtained synthetic intermediates. Our method generates products not easily obtained by other procedures and can be diverted to give products of a formal cycloaddition involving acetone. Given the sheer number of cyclopentane-bearing natural products and other complex structures, the use of reagent 1 as an annulation reagent seems promising.
The research described above resulted in our first peer-reviewed publication in
In a similar manner, the graduate students that performed the experiments above will benefit from having a high-profile publication on their CV. Naturally, during the course of these studies, they have also mastered basic and advanced organic chemistry techniques used routinely in organic synthesis labs. Moreover, in troubleshooting their experiments, they have developed valuable problem-solving and rational thinking skills.