Reports: ND651675-ND6: Towards spatially-resolved ultrafast imaging of individual reduced graphene oxide sheets
Masaru K. Kuno, University of Notre Dame
Graphene oxide (GO) is an important precursor in the production of chemically-derived graphene. During reduction, GO’s electrical conductivity and band gap change gradually. Doping and chemical functionalization are also possible, illustrating GO’s immense potential in creating functional devices through control of its local hybridization. Though this project, we show that laser-induced photolysis controllably reduces individual single-layer GO sheets. This has been established by following the laser-induced reduction of single layer GO in real time via correlated single sheet absorption and emission imaging.
Our initial study has reported the direct observation of photolytic GO reduction, its heterogeneous intrasheet kinetics, and mechanistic aspects of GO-to-rGO interconversion. We have also detailed correlations between the spatial location of where GO’s reduction begins and have rationalized the origin of its spatially heterogeneous chemistry. This has been accomplished through an analysis of real-time single layer GO photoreduction movies.
This data shows that GO photoreduction is characterized by unusual behavior in its emission, namely (a) initial emission quenching and blueshifting, followed by (b) subsequent (dramatic) enhancement of its emission quantum yield accompanied by large spectral redshifts and (c) eventual photobleaching accompanied by fluorescence intermittency (i.e. blinking). We assign these observations to three stages (referred to as regions 1, 2 and 3 in what follows) in the photoreduction life cycle of GO.
Specifically, by analyzing time-dependent trends in emission movies as well as by conducting detailed ensemble and single sheet control measurements, we have established that region 1 represents GO photoreduction to reduced graphene oxide (rGO). Region 2 is attributed to the subsequent fragmentation of rGO into molecular domains and region 3 represents the photobleaching and single molecule behavior of these fragments. We have also attributed photoreduction to the photolysis of oxygen containing functional groups in GO wherein dominant species within GO's basal plane are hydroxyl (OH) and epoxy (C-O-C) groups. We exclude a more commonly invoked photothermal reduction mechanism as estimated temperature changes within GO following the absorption of light are on the order of a Kelvin. This hypothesis has been corroborated through additional frequency and temperature-dependent measurements, described in our manuscript.
Obtained emission movies simultaneously enable us to establish the kinetics and energetics of GO photolysis through rate constant maps constructed for regions 1 and 2. Using Arrhenius plots, we find that extracted rate constants are associated with activation energies (Ea) of Ea1=0.20−0.22 eV in region 1 and Ea2=0.47−0.50 eV in region 2. The smaller activation energies characteristic of region 1, in turn, suggest that GO photoreduction is dominated by the photolysis-induced migration of basal plane OH. In this regard, theoretical OH migration activation energies are ~0.32 eV. Larger activation energies observed in region 2, by contrast, are consistent with direct OH dissociation (0.7 eV), C-O-C migration (Ea ~ 0.9 eV), and possibly C−O−C/C=O/COOH dissociation (>1 eV). Notably, the photolysis of these functionalities results in significant carbon loss through CO and CO2 evolution and would rationalize the eventual photodegradation of rGO into molecular domains. Consequently, our initial study has enabled us to establish a clear physical and mechanistic picture for GO photoreduction.
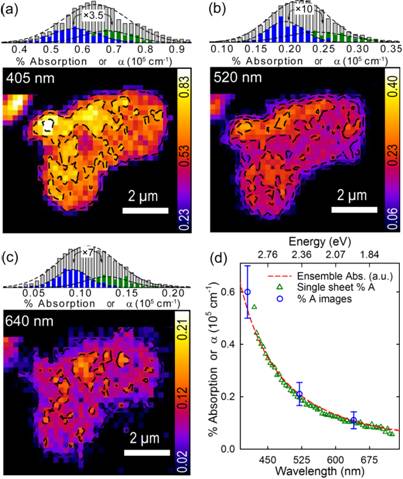
Next, in a second report, we have followed up this initial study by monitoring the photolytic reduction of single layer GO through concerted, spatially-resolved, absorption and emission microscopy. Acquired absorption/emission movies again illustrate the initial stages of GO reduction, its transition to rGO and its subsequent decomposition upon prolonged laser illumination. Subsequent analyses of these absorption movies have allowed us to establish native GO and rGO absorption coefficients (representative data shown below in Figure 1), their intrasheet distributions, and their spatial heterogeneities. Furthermore, an analogous kinetic analysis has allowed us to obtain spatially-resolved absorption-based rate constants and activations energies for GO photoreduction in regions 1 and 2. We find near identical photolytic activation energies that confirm our earlier emission-based estimates. These absorption measurements have also definitively established region 1 as GO photoreduction given the correlated rise in absorption (to ~2%) accompanied by a simultaneous drop in emission, resulting in a material which behaves like graphene (i.e. 2% absorption, no emission).
To summarize, our studies have revealed GO’s photoreduction life cycle for the first time. Detailed analyses of spatially-resolved kinetic data link local rate constants to activation energies and have enabled us to develop significant insight into GO’s underlying photoreduction mechanism. These studies have also established photolysis as the origin of GO’s photoreduction. On a broader level, the single layer absorption imaging we have developed is general and can be applied towards investigating the optical properties of other two dimensional materials, especially those that are non-emissive and which are invisible to current single molecule optical techniques.
Impact on career and on participating students
The current grant has been very helpful in advancing the PI’s career. Specifically, data obtained from this grant has enabled the PI to secure two additional grants from the Department of Defense (ARO: Colloidal syntheses of two dimensional titanium disulfide and CdSe nanosheets and their single sheet absorption spectroscopy; ARO DURIP: Intraband and intersubband absorption spectrometer for probing individual low dimensional nanostructures). These grants focus on the absorption microscopy/spectroscopy of single layer 2D materials such as TiS2, MoS2, MoSe2, and WSe2.
The grant has also supported two students recently (Yurii Morozov and Jixin Si). The first was a short term research visitor from Ukraine who eventually switched graduate programs and is now a graduate student at Notre Dame. The second is a student with a physics collaborator with whom we have worked with to better understand the observed blinking of rGO sheets. Jixin is currently writing a manuscript on this work which will be submitted shortly.
Representative image
Figure 1. Absorption images of single layer GO acquired at (a) 405 nm, (b) 520 nm and (c) 640 nm. Histograms above each image show the distribution of % absorption values and associated absorption coefficients for a GO ensemble and for two separate single layer sheets. (d) Single layer and ensemble GO absorption spectra overlaid with measured absorption coefficients.