Reports: DNI552769-DNI5: Electric Field-Induced Reactions in Fuel Cells From First Principles
Jean-Sabin McEwen, PhD, Washington State University
Solid oxide fuel cells (SOFC) are a promising technology for generating electrical energy by the conversion of chemical energy with very high efficiency, unique scalability, fuel flexibility, low emissions, and long-term stability. To control the ongoing reactions in such a device one can modify the selectivity of the catalyst itself, which oxidizes the fuel at the anode and dissociates O2 at the cathode. The selectivity of the catalyst in SOFCs will be altered by the presence of a large electric field of the order of 1 Volts per Angstrom. These high electric fields generated at the electrode-electrolyte interface are strong enough to induce the rearrangement of electronic orbitals of molecules, in particular at metal surfaces, leading to new phenomena, which can be summarized as field-induced electrocatalysis. In this way, this external electric field may stabilize the unstable molecule or may alter reaction pathways in heterogeneous catalysis. However, up until now, the field-induced effects on the mechanisms and kinetics of the heterogeneous reactions in SOFCs have remained largely uninvestigated.
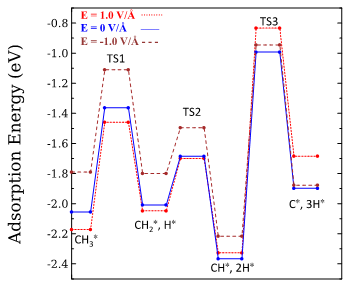
Fig.1. Energy pathway for the methyl dissociation to C and H on the Ni(111) surface in the presence and absence of an external electric field. All the energies are relative to the adsorption energy of a CH3 molecule on the Ni(111) surface.
To provide insight into the electrocatalytic reactions occurring at the electrode/electrolyte interface in SOFCs, an atomic level investigation of how an external electric field influences methane steam reforming on the Ni surface must be carried out using density functional theory calculations. In the past year and a half, we have already published two papers that related to the field-induced effects on the methane dissociation on both the Ni(111) and the Ni(211) surface. The results show that a high positive electric field of the order of 1 V/Å, could improve the performance of both the Ni(111) surface and the Ni(211) surface for the methyl species dissociation reaction, which is a necessary step of the methane steam reforming reaction. More specifically, a positive electric field strengthened the adsorption energy of the CH3 reactant, increased the energy barriers of the methyl dissociation and weakened the adsorption energies of C and H products on the Ni surfaces (see Fig. 1). These results suggest that a positive electric field will impede the formation of pure C atoms deposits on the Ni surfaces and consequently suppress coke formation and stabilize the industrial operations. This information can reveal important consequences for designing new chemical systems and enhancing the SOFCs technologies.
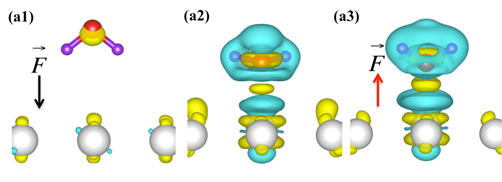
Fig.2. Differential charge density of a water molecule on a Ni(111) surface in the presence of a negative electric field (a1), a positive electric field (a2) and in the absence of an electric field (a2). The yellow and blue areas represent a gain and a electron loss, respectively.
Recently, we are focusing on the study of how an external electric field affects the interactions between water molecules and the Ni surfaces, which has widespread applications in many industrial catalytic processes (i.e. SOFCs). Due to the unique permanent dipole moment of a H2O molecule, it establishes the important role that H2O molecules will play in electrocatalysis. As one might expect, an external electric field has significant effects on the adsorption energy and geometries of water groups, the energy barriers, reaction heats, rate constants, and equilibrium constants of water dehydrogenation-formation reactions on both the Ni(111) and the Ni(211) surface (see Fig. 2).
Interestingly, the electric field effects on the water
reactions on the Ni surfaces are more significant than that of methyl
dissociation on the Ni surfaces from our previous works. Our results also showed
that the ![]() reaction
on the Ni(211) surface was the most energetically favorable one in the presence
of a negative electric field for water dehydrogenation. On the other hand, the
reaction
on the Ni(211) surface was the most energetically favorable one in the presence
of a negative electric field for water dehydrogenation. On the other hand, the
![]() reaction
on the Ni(111) surface in the presence of a negative electric field was competitive
for water formation processes. The manuscript of this research is currently in
preparation.
reaction
on the Ni(111) surface in the presence of a negative electric field was competitive
for water formation processes. The manuscript of this research is currently in
preparation.
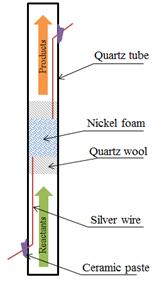
An experimental test has been also been designed based on similar experimental work by Yasushi Sekine, et al. to test the findings of our theoretical work. Sekine’s work utilized a high electrical potential and beds of loose catalyst particles in an open circuit (1 mm gap distance between the upper electrode and the catalyst bed) to test reactions of ethanol1,2 and methane3 under varying electrical fields. In order to test theoretical findings of methane species reacting on a pure nickel surface, a nickel foam catalyst from Goodfellow (Sigma Aldrich) is being used in a flow-through reactor constructed of quartz (see Fig. 3 and Fig. 4). Silver wires attached to the nickel foam provide a convenient method for introducing an electrical field of ±10 V. Current is minimized in the circuit by introducing a high resistance (11 MΩ). This allows for the application of a (nearly) pure voltage, a condition in the theoretical calculations. A gas chromatograph is used to characterize and quantify the product gases.
Fig. 3. One of the disks of nickel foam from Goodfellow (Sigma Aldrich) used as a catalyst. The disk itself is 1 cm in diameter, and the silver wire is attached with a conductive silver paste.
Fig 4. A schematic of the reactor used for these experiments. Total length of the quartz tube used is about 30 cm, with a 1 cm inner diameter. Quartz wool is used as a support to keep the catalyst bed in place.
References
(1). Y. Sekine, M. Tomioka, M. Matsukata, E. Kikuchi, Catal. Today, 2009, 146,
183-187.
(2). Y. Sekine, M.Haraguchi,M. Tomioka, M.Matsukata, E. Kikuchi, J. Phys. Chem. A,
2010, 114, 3824-3833.
(3). Y. Sekine, M. Haraguchi, M. Matsukata, E. Kikuchi, Catal. Today, 2011, 171,
116-125.