58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND451053-ND4: Cucurbiturils in Organic Reactions: Encapsulation and Stabilization of Key Transient Species for Supramolecular Catalysis
Eric Masson, PhD, Ohio University
1. Introduction. For the past two years, our group has been exploring Cucurbit[n]uril (CB[n])-contained reactions and conformational changes in aqueous medium (see sections 3.1 – 3.3). CB[n]s are pumpkin-shaped macrocycles bearing n glycoluril motifs linked by methylene bridges, two hydrophilic carbonylated portals and a hydrophobic cavity. Our group has also diversified its activities, and has been designing dynamic supramolecular polymers, which use CB[8] as "soft" non-covalent connectors between monomeric units (see section 3.4).
2. Impact. This year, PRF support allowed us (1) to publish three articles (one in the European Journal of Organic Chemistry, see section 3.1, and two in Organic and Biomolecular Chemistry, see sections 3.2 and 3.3 – the study described in section 3.3 was selected for the cover image of the issue) and (2) to have a pending article in JACS (currently under revision, see section 3.4). The PRF-funded postdoctoral associate is a co-author on three of these articles, including the one in JACS. In addition, two graduate students, whose supplies were purchased with PRF funds, have generated enough results to prepare at least two additional manuscripts. PRF funding had a formidable impact on our research activities, and is currently allowing the preparation of two proposals to the National Science Foundation during the fall submission window (Macromolecular, Supramolecular and Nanochemistry, as well as Division of Materials).
3. State of Research
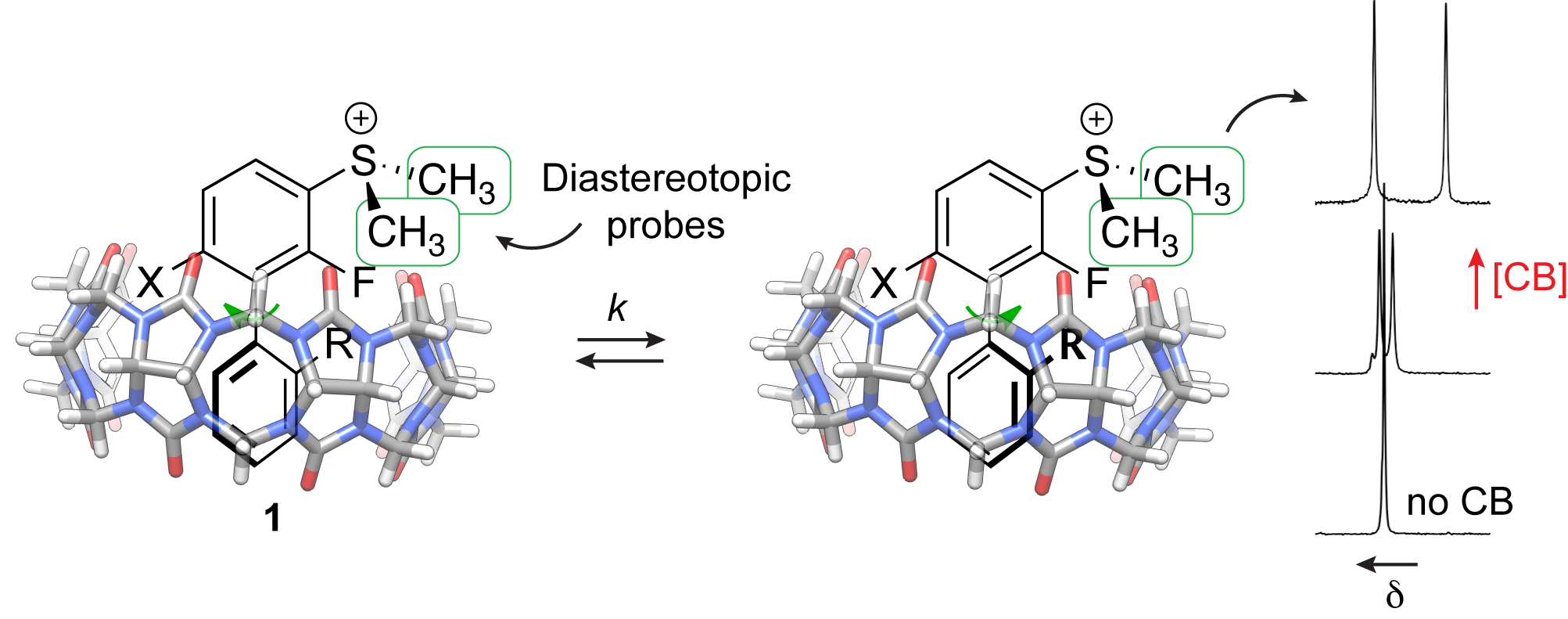
3.1. CB[n]s as tools to determine the torsional barrier of substituted biphenyls. We showed that the torsional barrier of biphenyls 1, which bear a prochiral dimethylsulfonium group at their 3-position, could be determined by variable temperature 1H NMR spectroscopy only after encapsulation into CB[7] or CB[8]; the macrocycles triggered the splitting of the two methyl signals. The key finding of this study is that confinement of the biphenyl units into the macrocycles amplifies the dissymmetry caused by the various ortho- and ortho'-substituents. We also determined that CB[n] encapsulation has a very limited impact on the torsional barriers, and we compared the latter with calculated values obtained using a highly accurate benchmarked density functional method (see section 3.2). To the best of our knowledge, this study is the first case of CB[n] use as an analytical accessory to access coveted kinetic parameters such as these torsional barriers. It is also anticipated to be applicable to a wide range of bi(het)aryl derivatives.
3.2. Torsional barriers of substituted biphenyls calculated using density functional theory: a benchmarking study. When attempting to compare the activation barriers of substituted biphenyls inside CB[n]s (see section 3.1) with activation energies obtained in silico, we realized that density functionals and basis sets had never been benchmarked against a large set of biphenyl derivatives and a wide interval of barriers. Therefore, we calculated the barriers of torsional isomerization of 13 substituted biphenyls (ranging from 7.4 to 44 kcal/mol), using 9 density functionals (some combined with D and D3 corrections for dispersive interactions), and we compared the results with experimental data. The B3LYP–D, B97–D and TPSS–D3 functionals were identified as the most promising methods (thereby indicating that attractive dispersive interactions between substituents cannot be neglected), and were used to determine the torsional barriers of 33 other substituted biphenyls with known Gibbs energies of activation (6.0 to 45 kcal/mol). Throughout the 46-member ensemble, results were very accurate relative to experimental values (mean deviation between –0.38 and 0.24 kcal/mol), and narrow distributions of errors were obtained (mean absolute deviations ranging from 0.61 to 0.75 kcal/mol). If the procedure described in this study is applied to other biphenyl derivatives, one can be 99% confident that 95% of their calculated barriers will be within 2.4 kcal/mol of experimental data. When barriers lower than 30 kcal/mol are considered, this tolerance shrinks to 1.8 kcal/mol and rivals analytical accuracy.
3.3. Subtle "supramolecular buttressing effects" in Cucurbit[7]uril/guest assemblies. The term "buttressing effect" was proposed by Westheimer in 1950 to describe the "cascade" impact of meta-substituents on the rigidity of ortho-substituents, which in turn affect the torsional barriers of substituted biphenyls. Our group realized that the concept of chemical buttressing had never been extended to supramolecular systems, and we defined it as the alteration, by a neighboring unit, of a substituent effect on intermolecular recognition. Using biphenyl derivatives 2 as model scaffolds, we determined that as free species, those bearing 4-H and 4-F substituents are present as approximately equimolar mixtures of syn and anti-conformers, while the biphenyl scaffold with a 4-CH3 group adopts the anti-conformation exclusively. The 3-dimethylsulfonium substituents then interact with one of the carbonylated portals of Cucurbit[7]uril (CB[7]), and their conformations affect the position of the guests inside the cavity of the macrocycle, thereby validating our "supramolecular buttressing" model.
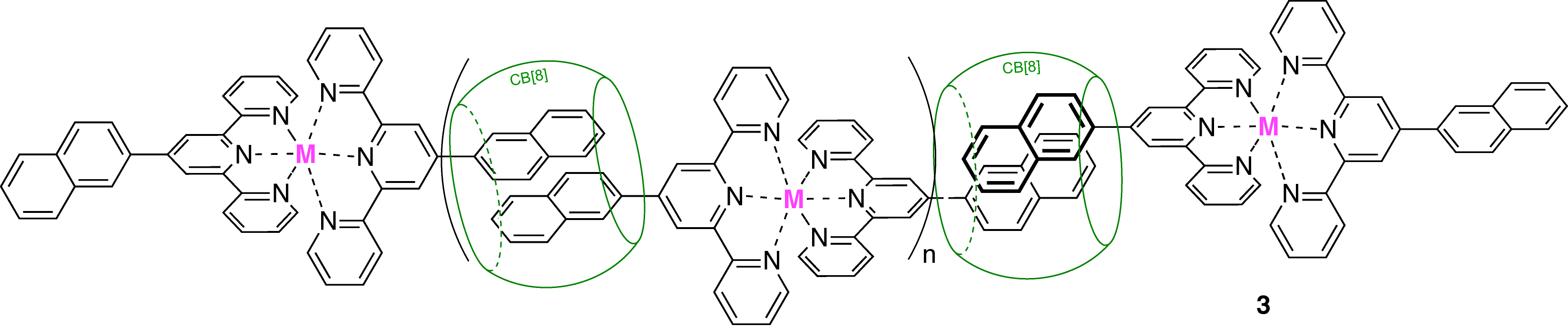
3.4. Stabilization of Cucurbituril/guest assemblies via long-range Coulombic and CHáááO interactions. Coulombic interactions between positive units and the portals of CB[n]s can be caused by direct contact between a metallic or organic cation and the carbonyl laced portal of the macrocycle, or by water mediation between the two units as it is often the case with transition metals and clusters in the solid state. In this study, we showed for the first time that Coulombic interactions between a metal and CB[n]s can be mediated by an organic ligand (see terpyridine derivative 3, for example), and that even in the second coordination sphere, CB[n]s form remarkably strong CHáááO hydrogen bonds with the ligand surrounding the metallic core. While [3]pseudorotaxanes are formed upon addition of CB[7] to metal¥ligand2 complexes, dynamic oligomers are obtained in the presence of stoichiometric amounts of CB[8], which acts as a "soft", non-covalent connector between metal/terpyridine complexes.
Copyright © 2014 American Chemical Society