58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI952190-DNI9: Linking Fractional Free Volume to Gas Solubility and Membrane Permeability in Ionic Liquids
Jason E. Bara, PhD, University of Alabama (Tuscaloosa)
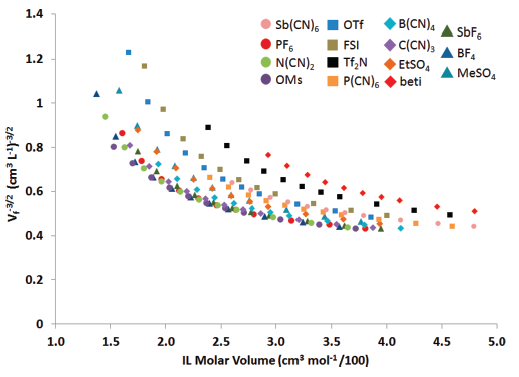
Prior to being awarded the ACS-PRF DNI, but after the grant submission, we had successfully completed a modeling study wherein the software package COSMOTherm was employed to estimate fractional free volume (FFV) across various ionic liquids (ILs) with systematically varied structures.1 Specifically, we examined the relationship between 1-n-alkyl-3-methylimidazolium cations with various anions (e.g. [Cnmim][X] ILs) and identified that the experimentally observed selectivities for CO2/N2 and CO2/CH4 in these ILs can be described as a simple function of the IL free volume to the (-3/2) power (Figure 1).
Figure 1: Plot of IL free volume to the (-3/2) power for [Cnmim][X] IL families relative to IL molar volume.
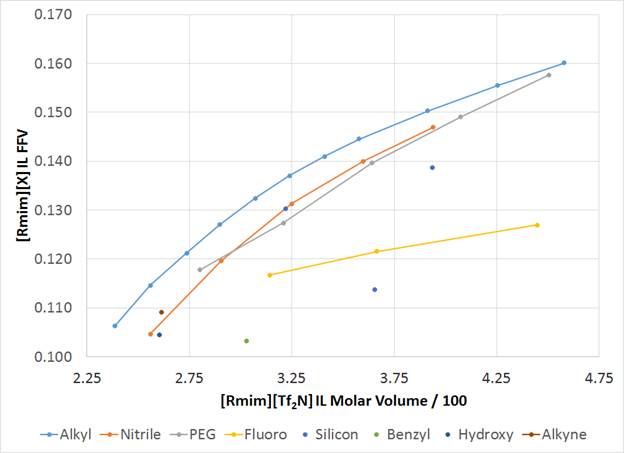
We are now building upon this concept in the analysis of ILs with various chemical functionalities that are not n-alkyl chains. Specifically, we have studied 1-R-3-methylimidazolium cations with Tf2N anions (i.e. [Rmim][Tf2N]) where ‘R' is a polar group such as oligo(ethylene glycol), nitrile-terminated alkyl, or hydroxyl-terminated alkyl. The results of these simulations show that these non-alkyl ‘R' groups reduce FFV relative to [Cnmim][X] ILs with similar sized side chains (Figure 2). IL FFV Is purely a calculation based on cation and anion sizes and occupied volume. It requires no inputs other than the ion structures within the COSMOTherm package (Figure 3).
Figure 2: Plot of FFV in [Rmim][Tf2N] ILs relative to IL molar volume.
Figure 3: Example structures of
functionalized cation and anion generated to
calculate FFV within COSMOTherm.
Thus, this methodology can be used as a guideline to confirm that the inclusion of polar, rather than n-alkyl groups, is a highly justifiable approach to improving gas solubility selectivities of CO2/N2 and CO2/CH4. A manuscript discussing these new studies has recently been submitted to the Journal of Chemical Thermodynamics as part of their forthcoming special issue on the 5th Congress on Ionic Liquids (COIL-5) which several members of our group attended with the aid of travel funds from the DNI award in April 2013.
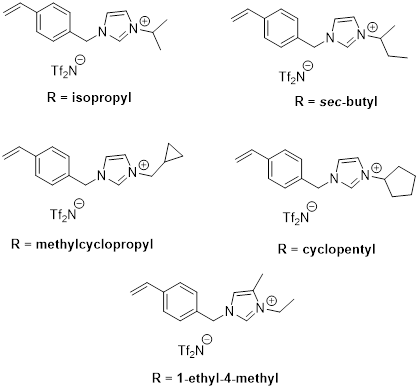
Furthermore, we have completed experiments on a new set of polymerized ILs (poly(ILs)) with branched and cyclic groups as membranes for the separation of CO2/N2 and CO2/CH4. These poly(ILs) are built from functionalized monomers represented by Figure 4. The results in Table 1 show that branching, cyclization or distribution of alkyl groups improves CO2 selectivity compared to n-alkyl analogues. These results are the focus of a manuscript that we are currently preparing to submit to Journal of Membrane Science
Figure 4: Structures of new IL monomers with various ‘R' groups studied as polymer gas separation membranes.
Table 1: Results on poly(IL) gas separation membranes that employ cyclic, branched or distributed alkyl groups.
‘R' Group(s)
| P CO2
| P N2
| P CH4
| CO2/N2
| CO2/CH4
|
isopropyl
| 10.37
| 0.33
| 0.35
| 31.09
| 29.75
|
sec-butyl
| 13.58
| 0.39
| 0.55
| 34.63
| 24.90
|
methylcyclopropyl
| 7.94
| 0.24
| 0.32
| 33.17
| 24.99
|
cyclopentyl
| 6.65
| 0.19
| 0.25
| 34.22
| 26.72
|
1-ethyl-4-methyl
| 13.81
| 0.34
| 0.38
| 40.33
| 36.22
|
During the course of this work, we have also discovered a unique swelling behavior of poly(IL) materials in organic solvents. In a stunning example, we have observed a poly(IL) based on the simply monomer in Figure 4 gain over 200 times it mass in DMSO. This work will be the subject of a manuscript submitted to Chemistry of Materials and can reveal much about the internal structures of poly(IL) membranes and how they are structured to adapt or accommodate liquid or gas solute molecules such as CO2.
We plan to continue studying new IL designs in the next year of this project to develop further evidence of the relationships between fractional free volume and IL structures.
1. Shannon, M. S.; Tedstone, J. M.; Danielsen, S. P. O.; Hindman, M. S.; Irvin, A. C.; Bara, J. E. Free Volume as the Basis of Gas Solubility and Selectivity in Imidazolium-Based Ionic Liquids. Industrial & Engineering Chemistry Research 2012, 51, 5565-5576.
Copyright © 2014 American Chemical Society