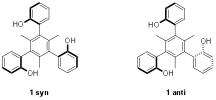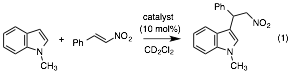58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND452655-ND4: Hydrogen Bond Catalysis and Anion Molecular Recognition
Steven Kass, University of Minnesota
Hydrogen bond catalysis is exploited in nature and has been modeled by chemists. Thioureas are the most commonly used hydrogen bond catalysts, and they owe a large part of their effectiveness to the fact that they have two N–H bonds. That is, they can serve as a two hydrogen bond donor. In select cases enzymes make use of three hydrogen bonds and we wondered if this could lead to the development of a new catalyst class. Moreover, oxygen acids are more acidic than nitrogen acids in general, so we prepared the rigid triols below in both rotameric forms (1syn and 1anti); they do not interconvert except at high temperature. We found that the syn species, which has all three OH's on one side of the plane defined by the central aromatic ring and can act as a three hydrogen bond donor, is a more active catalyst than the anti compound, which can only serve as a two hydrogen bond donor. In the Friedel-Crafts reaction illustrated in equation 1 we found that ksyn/kanti = 160.
We also have discovered that electron withdrawing groups can be used in combination with hydrogen bond networks to afford strong Bronsted acids as illustrated below:
Copyright © 2014 American Chemical Society